Figures & data
Table 1 Composition of Baclofen-Meloxicam ODTs Prepared by Direct Compression Using the Selected Co-Processed Excipients
Table 2 Composition of Baclofen-Meloxicam ODTs Using Freeze-Dryer with Different Ratio of Matrix Former and Collapsing Agents
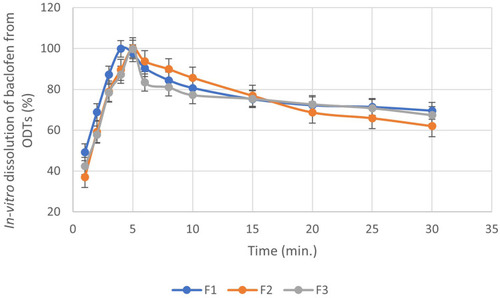
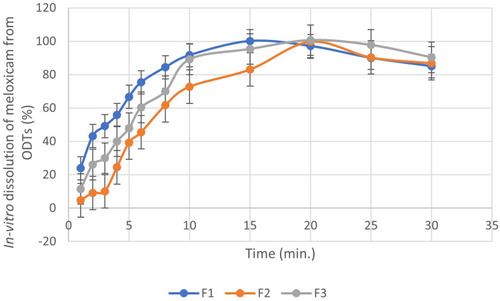
Table 3 Physical Evaluation of the ODTs Using Different Co-Processed Excipients
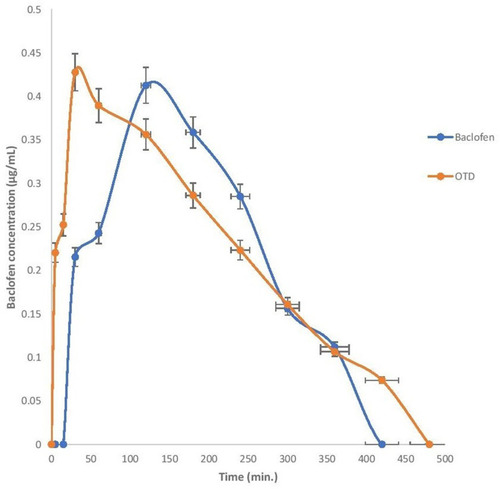
Figure 1 FT-IR spectrum of (A) meloxicam, (B) baclofen, (C) physical mixture of meloxicam and baclofen, (D) Prosolv ODT, (E) F-melt, (F) Pharmaburst 500, (G) physical mixture of meloxicam and Prosolv ODT, (H) physical mixture of meloxicam and F-melt, (I) physical mixture of baclofen and Prosolv ODT, (J) physical mixture of baclofen and F-melt, and (K) physical mixture of baclofen and Pharmaburst 500, (L) physical mixture of meloxicam and Pharmaburst 500.
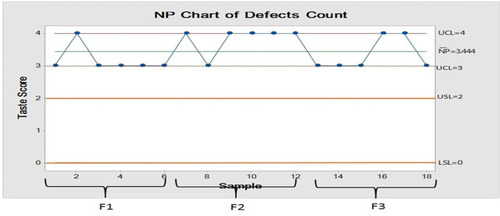
Table 4 Bitterness Taste Score of Baclofen-Meloxicam ODTs by Volunteers on Three Successive Days
Table 5 VOC Experience vs Expectation
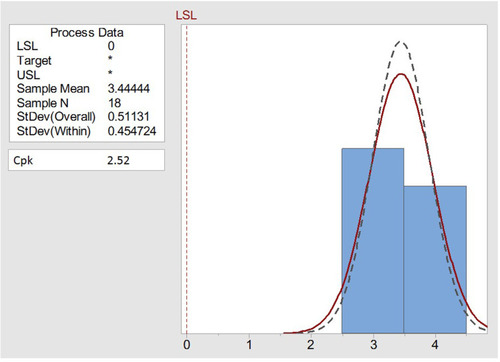
Table 6 Physical Evaluation of the Improved Baclofen-Meloxicam ODTs After Applying Six Sigma Using Lyophilization Technique
Figure 15 Differential scanning calorimetry thermograms of the raw materials and the optimal ODT formula (F4).
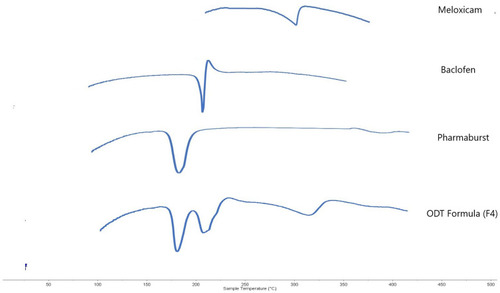
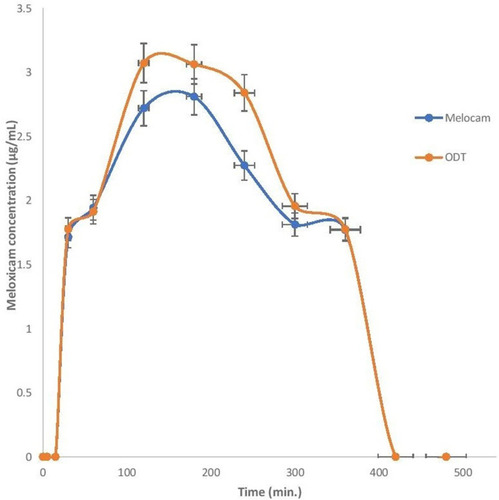
Table 7 Summary of the Pharmacokinetic Parameters of Meloxicam and Baclofen Following the Administration of Commercial Oral Tablets and the Baclofen-Meloxicam ODT