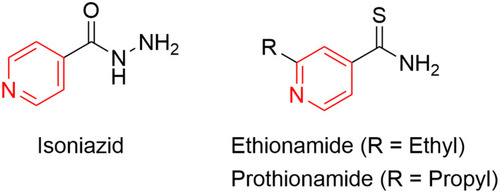
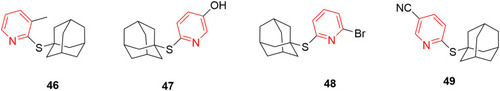
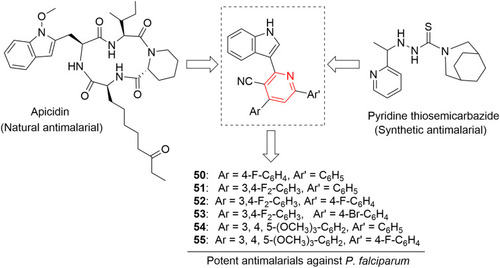
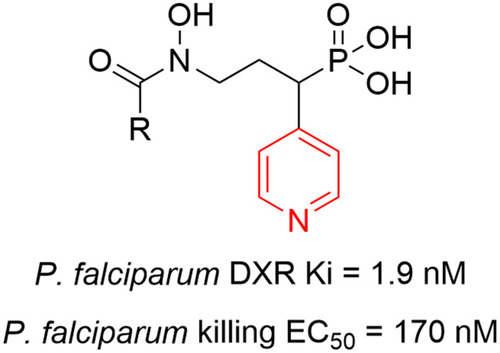
Figures & data
Figure 2 Substitution-type analysis of pyridine- (A) and dihydropyridine (B)-containing FDA-approved drugs.
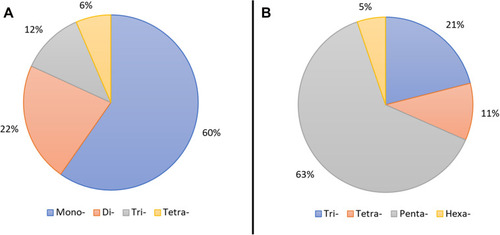
Figure 3 Publications on pyridine- and dihydropyridine-containing compounds, 2010–2020 (source: Scopus and SciFinder).
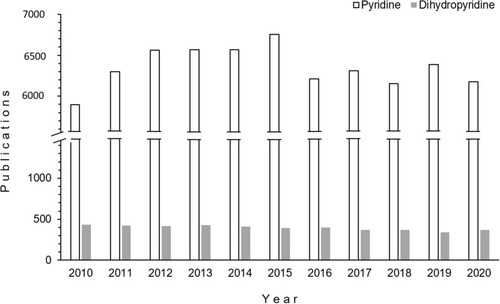
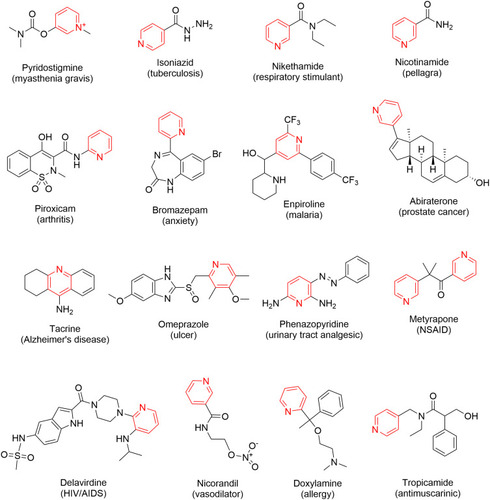
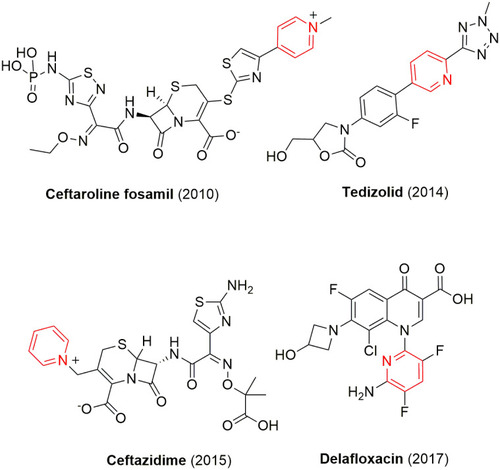
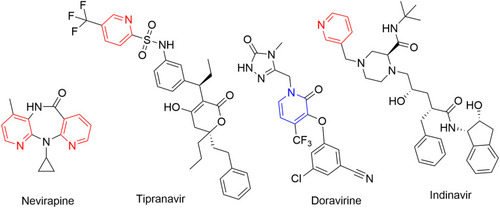
Table 1 Commercially available pyridine- and/or dihydropyridine-containing drugs and their applications
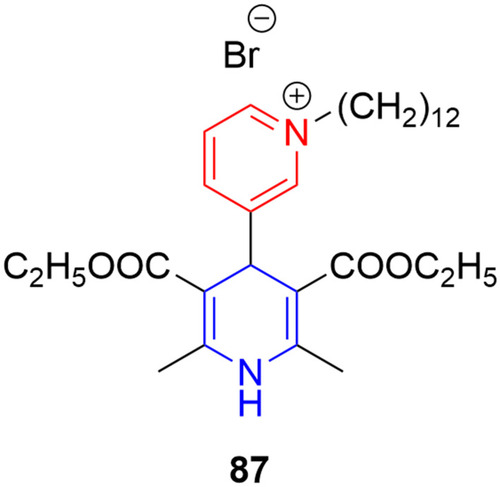
Figure 10 Dodecylpyridinium moiety containing dihydropyridines with potent calcium antagonism in the A7r5 cell line.
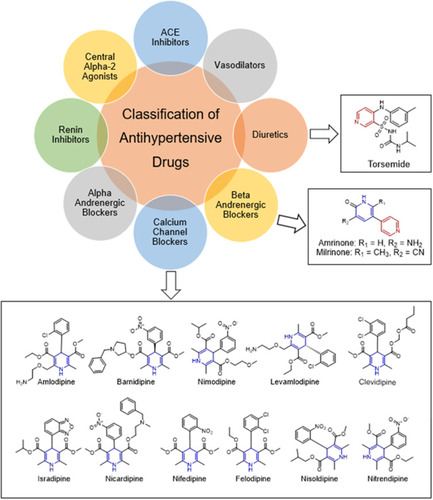
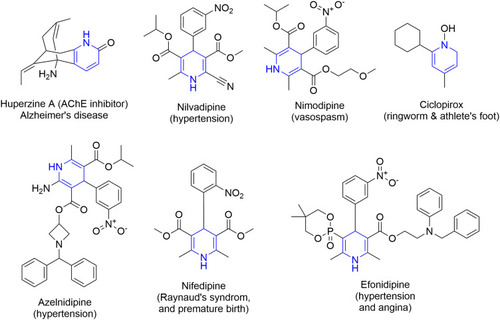
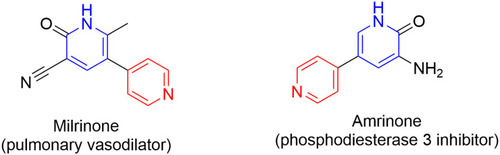
Figure 11 FDA-approved drugs containing pyridine or dihydropyridine scaffolds for the treatment of hypertension.
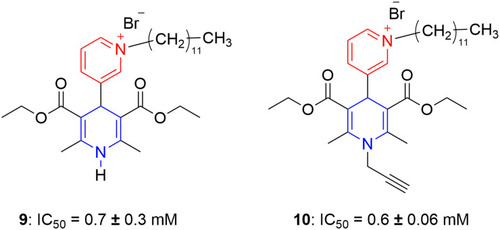
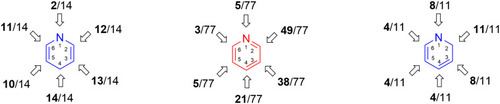
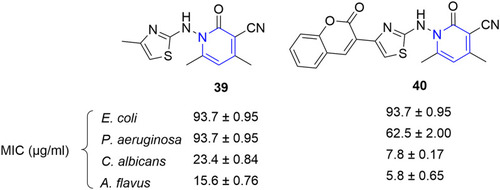
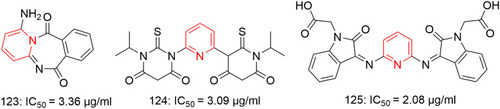
Figure 21 Pyrazolo[3,4-b] pyridine–bearing compounds with significant effect against various Gram-positive and Gram-negative bacterial strains.
![Figure 21 Pyrazolo[3,4-b] pyridine–bearing compounds with significant effect against various Gram-positive and Gram-negative bacterial strains.](/cms/asset/06e76954-5cfe-4b46-afc9-993c08649482/dddt_a_12184259_f0021_c.jpg)
Figure 24 Highly potent antitubercular compounds (43–45) with MIC values (µg/mL) against M. bovis BCG.
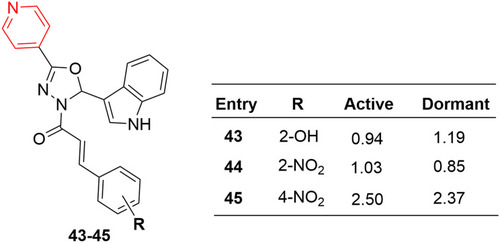
Figure 30 Pyridine–furan hybrid compounds with 50% reduction in viral titer against adenovirus 7 strain.
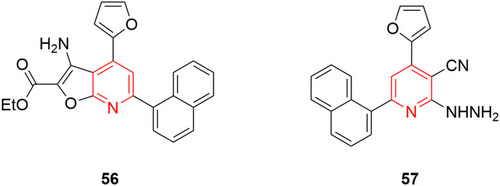
Figure 35 FDA-approved oxicam-class NSAIDs for musculoskeletal disorders, such as osteoarthritis and rheumatoid arthritis.
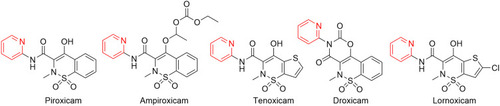
Figure 37 Indolyl pyridines (67–68) and dihydropyridine-containing compounds (69–71) with remarkable anti-inflammatory activity in animal models.
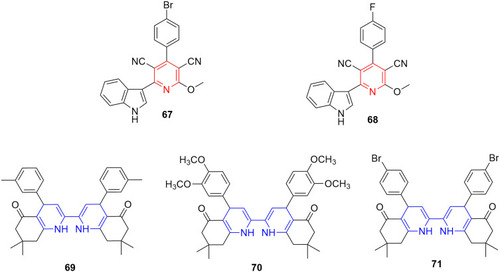
Figure 38 Thienopyridine derivatives (72–75) with anti-inflammatory and immunomodulatory profiles. IC50 values correspond to inhibition of NO production on murine RAW264.7 macrophages.
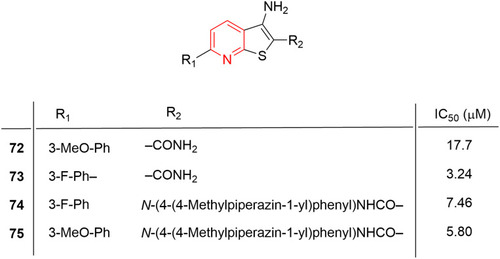
Figure 42 Pyridine- or dihydropyridine-containing drug-repurposing candidates for treatment of neurodegenerative diseases.
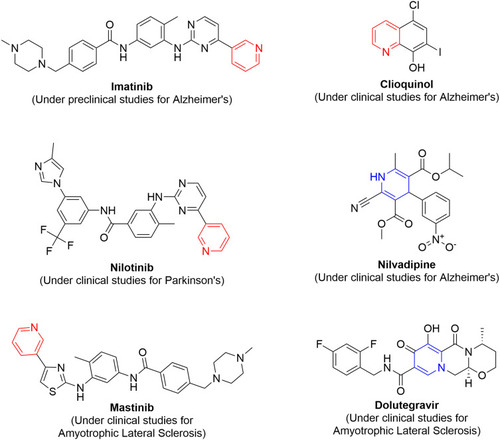
Table 2 Summary of neurogenic/neuroprotective compounds with pyridine or dihydropyridine scaffolds
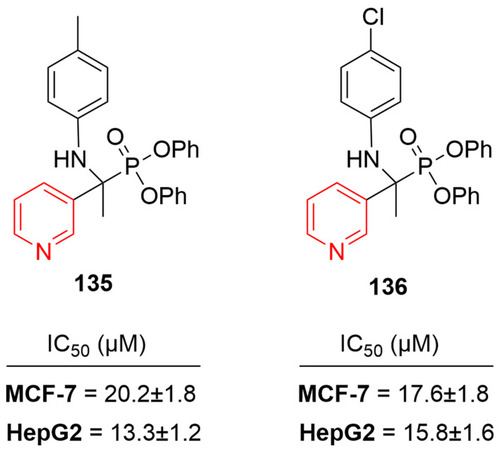
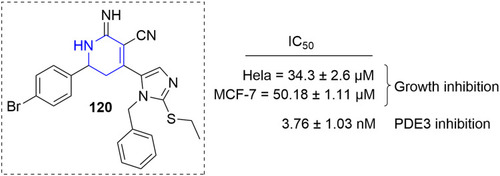
Figure 54 Pyridine–thiazole hybrids with remarkable anticancer effect in MCF7 breast adenocarcinoma.
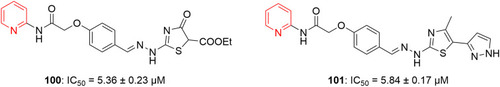
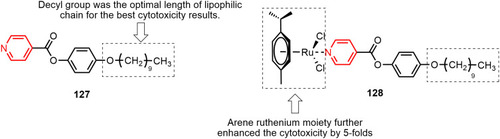
Figure 58 1,4-Dihydropyridine-containing benzylpyridinium moieties with remarkable anticancer activity.
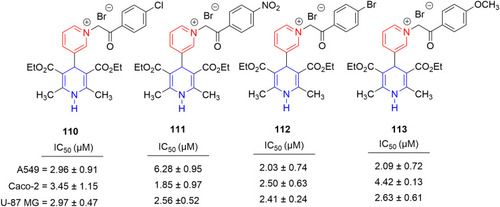
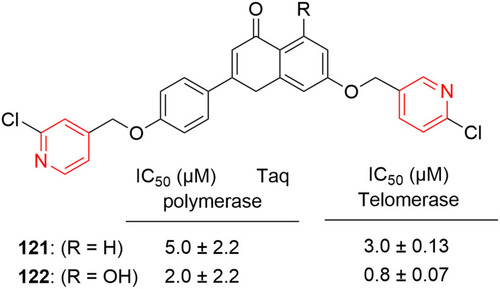
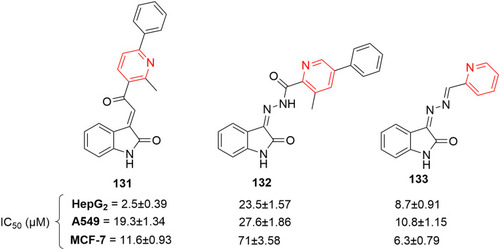
Figure 64 Pyridine–pyrimidine hybrid ring system containing compound 126 with inhibitory effects against NCI60 cell lines.
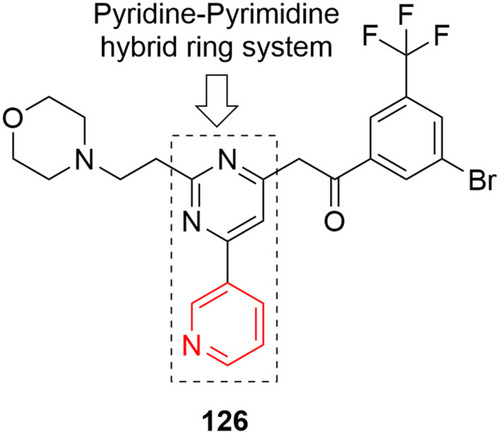
Figure 66 p-cymene–ruthenium complex 130 with submicromolar anticancer activity against ovarian cancer cell lines.
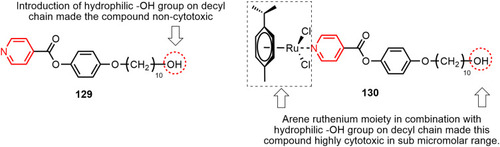

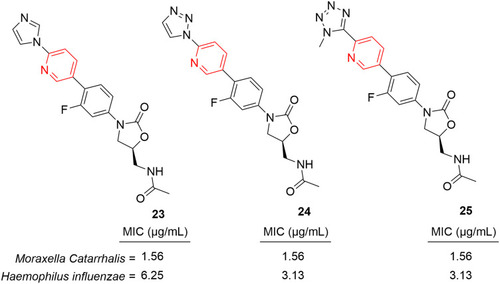
![Figure 20 Oxazolo[4,5-b]pyridines containing antibacterial agents with remarkable activity.](/cms/asset/95eecae4-3e12-4852-8bb4-807298d39cd2/dddt_a_12184259_f0020_c.jpg)
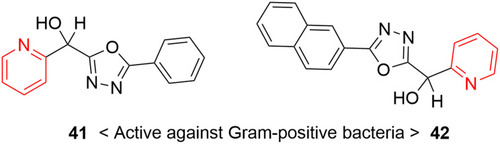
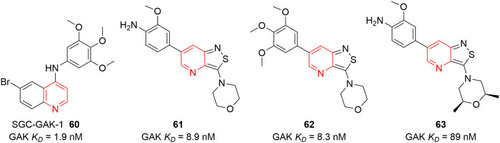
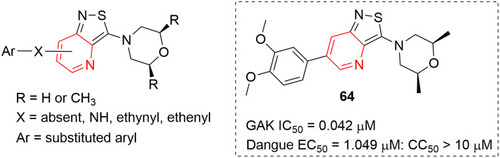
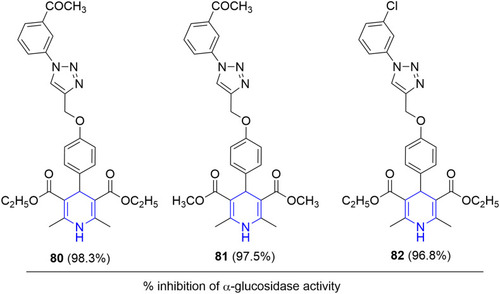
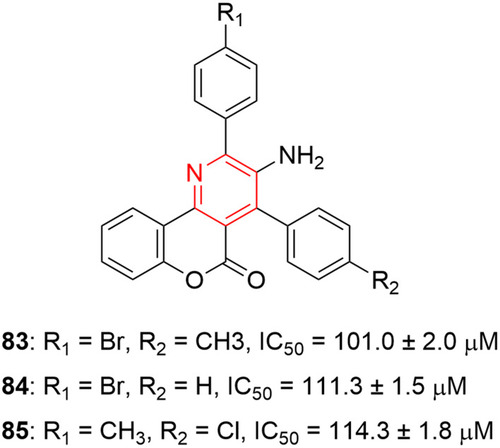
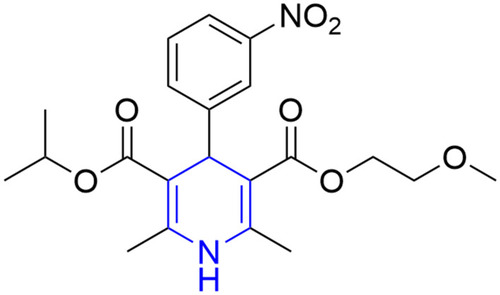
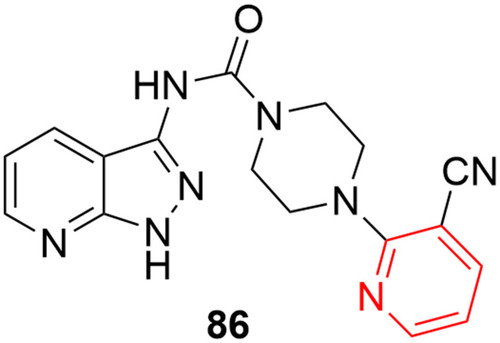
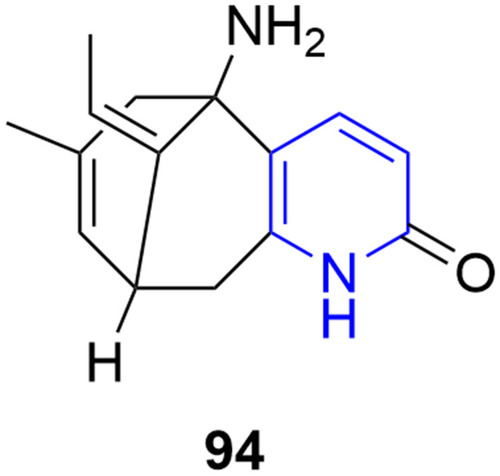
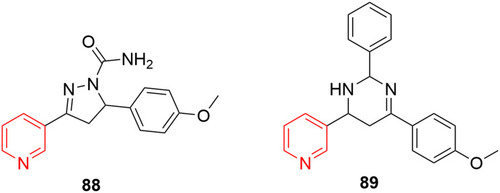
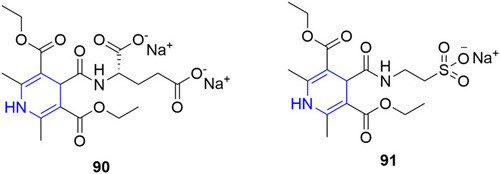
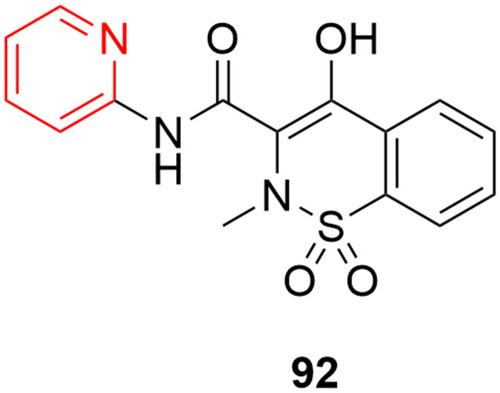
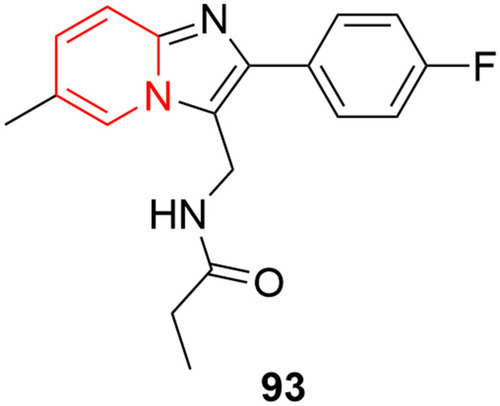
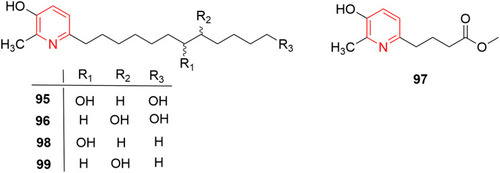
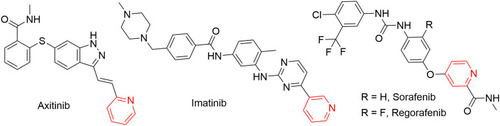
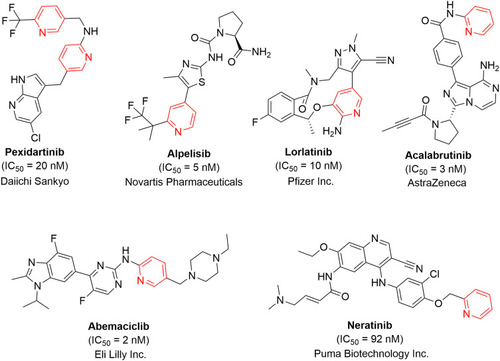
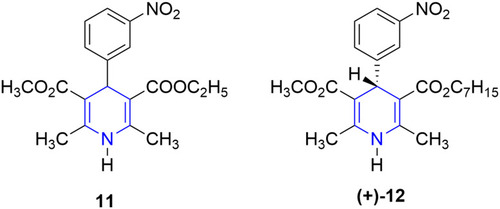
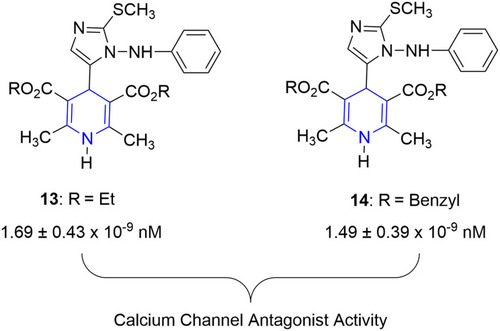
![Figure 55 Pyrazolo[3,4-b] pyridine- and dihydropyridine-derived compounds.](/cms/asset/1d125fec-9a2e-4b49-ac4b-5a865bb79c48/dddt_a_12184259_f0055_c.jpg)
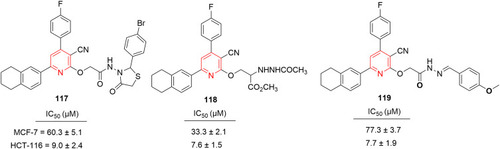
![Figure 68 [1,2,4]Triazolo[1,5-a]pyridinylpyridine–containing highly potent anticancer agent.](/cms/asset/37a0efd9-d4f1-4bb6-8808-3cf6b3840fca/dddt_a_12184259_f0068_c.jpg)