Figures & data
Table 1 Composition of various formulations of SEDDSs for simvastatin
Table 2 Composition of different formulations of S-SEDDSs for simvastatin
Table 3 Composition of different formulations of simvastatin self-emulsifying tablets
Table 4 Solubility of simvastatin in oils and their combination with Tween 60
Figure 2 (A) FTIR spectra of pure simvastatin, (B) simvastatin solution prepared in a mixture of olive oil and Tween 60, and (C) simvastatin solution prepared in castor oil and Tween 60.
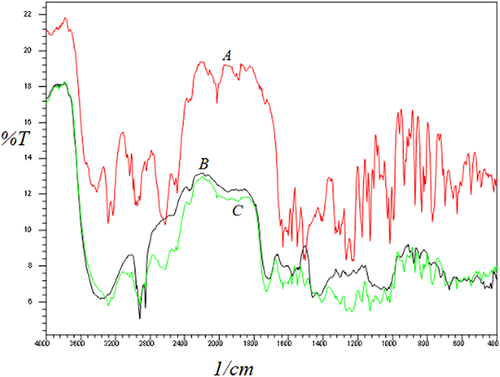
Table 5 Emulsification time and cloud point of self-emulsified formulations
Table 6 Results of precompression evaluation of powder blends for different formulations of SEDDS-based tablets
Table 7 Physical parameters and mechanical strength of SEDDS-based tablets