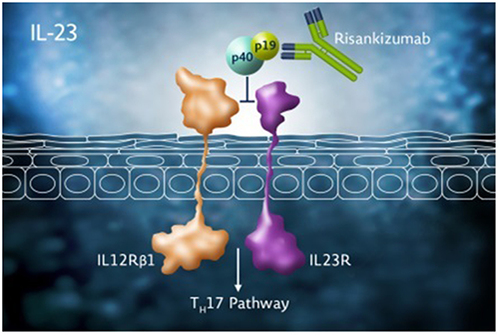
Figures & data
Figure 2 (A–C) Risankizumab on-body injector (used with permission from AbbVie, Inc.) (A). On-body injector front view (B). On-body injector back view (C). Prefilled cartridge.
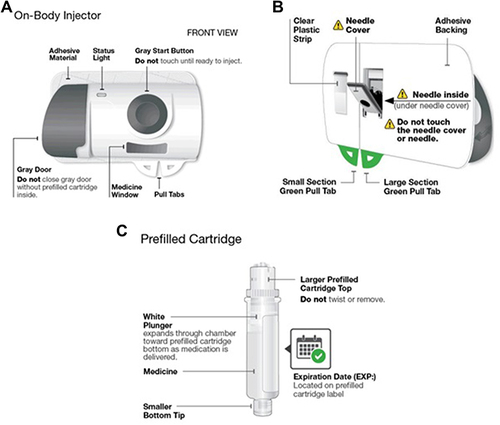
Table 1 Risankizumab Clinical Trial Results for Co-Primary Endpoints of Clinical Remission and Endoscopic Response in Induction (ADVANCE and MOTIVATE) and Maintenance (FORTIFY)
Table 2 Adverse Events for Patients on FDA Approved Dosing for Risankizumab Compared to Placebo in Phased 3 Trials for Risankizumab