Figures & data
Figure 1 Clinical and histological features of ulcerative colitis (UC) and causal factors influencing UC risk. Common diagnostic criteria of UC include both clinical and histological features. The main causes of UC are individual genetic background as well as environmental factors, which may alter/synergize with the genetic/epigenetic makeup.
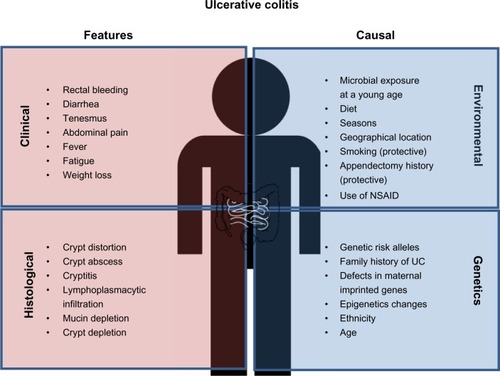
Figure 2 Mouse models of inflammatory bowel disease (IBD). Many mouse models are currently available that mimic the two major subtypes of IBD (Crohn’s disease [CD] and ulcerative colitis [UC]). Specific mouse models with characteristics of CD and/or UC are summarized.
![Figure 2 Mouse models of inflammatory bowel disease (IBD). Many mouse models are currently available that mimic the two major subtypes of IBD (Crohn’s disease [CD] and ulcerative colitis [UC]). Specific mouse models with characteristics of CD and/or UC are summarized.](/cms/asset/2ab05c75-3765-4552-85fe-07194136f4c7/dddt_a_40107_f0002_b.jpg)
Figure 3 Ulcerative colitis (UC) models that address the specific framework of disease pathology. Invertebrate (eg, Caenorhabditis elegans, drosophila) and vertebrate (eg, zebrafish, mouse) models have their respective advantages in the study of UC pathogenesis. Different models can be utilized and carefully chosen to more appropriately address particular questions, mainly altered enteric microbial membership, intestinal epithelial dysregulation, and aberrant immune responses.
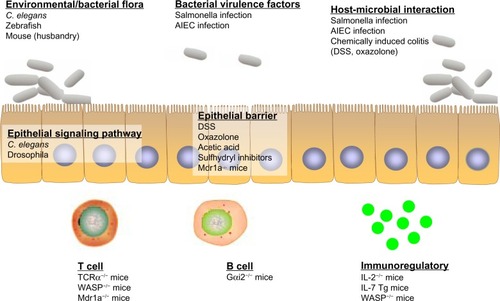
Figure 4 Current and emerging drug/therapy for ulcerative colitis (UC) treatment. Current treatments of UC mainly function by immunosuppression. Emerging therapies of UC have potential to target three major layers of UC dysfunction, including restoration of normal intestinal microbial flora, promotion of epithelial health (restitution of epithelium), and suppression of immunological cell trafficking and activation, with potentially fewer side effects compared with current available treatments.
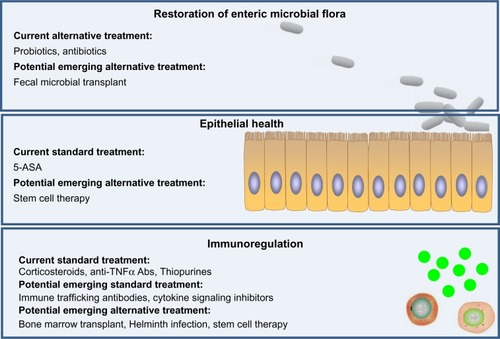
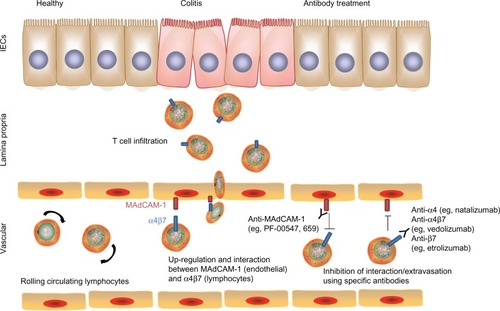
Figure 5 Blocking of immune cell trafficking to the colon. Upregulation of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on intestinal vascular cells and expression of integrins (eg, α4β7) on lymphocytes promote interaction and adhesion of lymphocytes onto the intestinal endothelium and subsequent extravasation into colonic tissues. Blocking MAdCAM-1 or α4β7 using specific antibodies disrupts this interaction and control of colitis severity.