Figures & data
Figure 1 A normal neuron (left) and an Alzheimer’s disease-affected neuron (right).
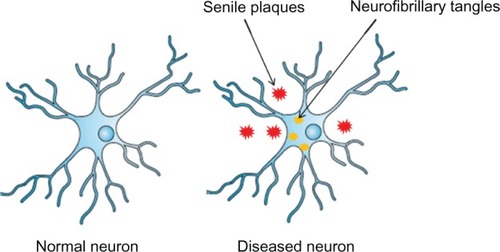
Figure 2 Hypothetical pathogenetic steps of the amyloid cascade hypothesis.
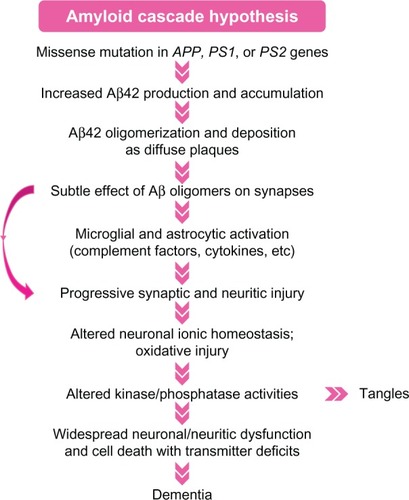
Figure 3 The mechanism of RNA interference.
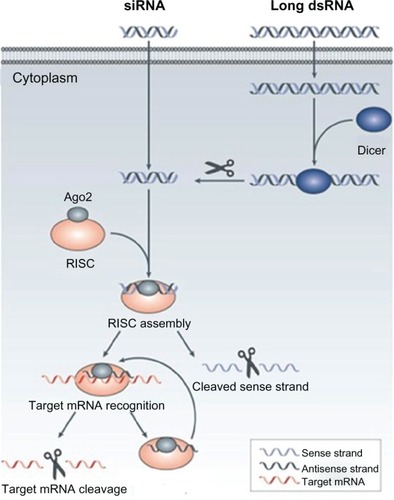
Table 1 Comparison of RNAi with traditional pharmaceutical drugs
Figure 4 Proteolytic processing of APP by á-, ß-, and ã-secretase.
Abbreviation: APP, amyloid precursor protein.
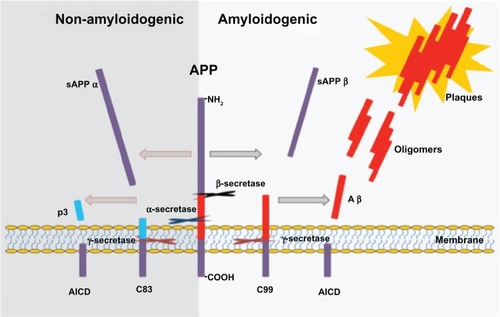
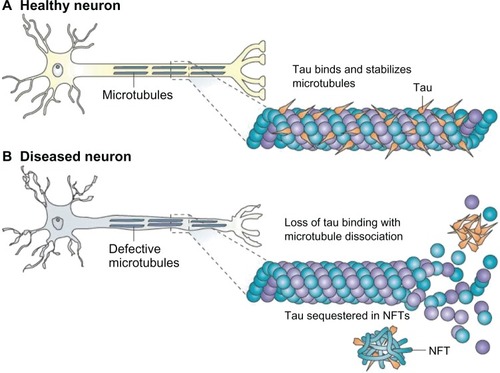
Figure 5 (A) Tau facilitates microtubule stabilization within cells and is particularly abundant in neurons. (B) it is thought that tau function is compromised in Alzheimer’s disease and other tauopathies.
Notes: This probably results from both tau hyperphosphorylation, which reduces the binding of tau to microtubules, and the sequestration of hyperphosphorylated tau into neurofibrillary tangles (NFTs), which reduces the amount of tau that is available to bind microtubules. The loss of tau function leads to microtubule instability and reduced axonal transport, which could contribute to neuropathology. Copyright © 2009. Nature Publishing Group. Reproduced with permission from Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat Rev Drug Discov. 2009;8(10):783–793.Citation97