Figures & data
Figure 1 Study design. Placebo-treated subjects were followed for at least 28 days until unblinding permits in each dose group. PK sampling time was up to 140 days for suramin treatment cohorts to better characterize the terminal phase, given the long elimination half-life based on emerging data.
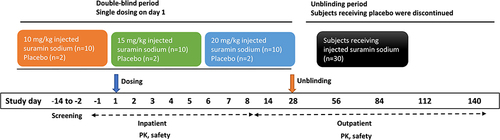
Table 1 Baseline Characteristics of the Randomized Study Population (n = 36)
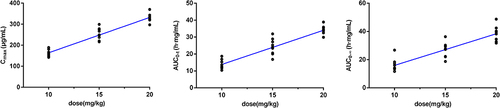
Table 2 Pharmacokinetic Parameters of Suramin After Single-Dose Administration of Injected Suramin Sodium in Healthy Subjects
Figure 2 Mean concentration–time curve with standard deviation for suramin in healthy human subjects after a single dose.
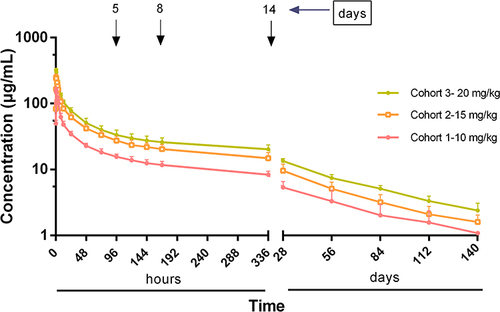
Table 3 Summary of Adverse Events