Figures & data
Figure 1 SGLT1 is involved in the pathological process of myocardial injury. Under some conditions, such as high glucose, ischemia/reperfusion injury, and pressure and volume overload, the expression of SGLT1 are increased in vascular endothelial cells, cardiomyocytes, and cardiac fibroblasts, which participate in the pathological processes of myocardial damage such as mitochondrial dysfunction, oxidative stress, and fibrosis, ultimately leading to cardiac remodeling, systolic and diastolic dysfunction, and the occurrence of heart failure.
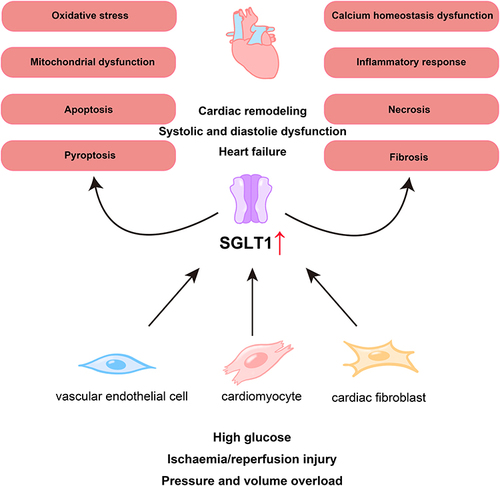
Figure 2 Sites and functions of SGLT1 and SGLT2 in kidney and of SGLT1 in small intestine, heart and brain. SGLT1 is mainly expressed in the small intestine and also expressed in the kidney, heart, and brain, while SGLT2 is mainly expressed in the kidney. In the kidney, both SGLT1 and SGLT2 are responsible for glucose reabsorption. In the small intestine, SGLT1 is responsible for the absorption of glucose and galactose from the gut lumen. In the heart and brain, SGLT1 plays an important role in energy metabolism.
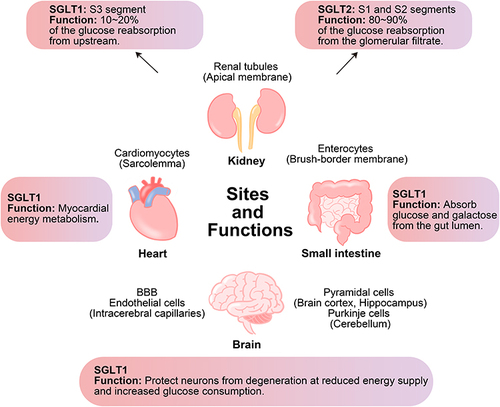
Table 1 Current/Under Development SGLT2, Dual SGLT1/SGLT2, and SGLT1 Inhibitors
Figure 3 Potential molecular mechanisms of SGLT1 inhibition in diabetes-related heart injury. SGLT1 inhibition can reduce cardiomyocyte fibrosis and apoptosis through JNK and p38 MAPK pathways, as well as block Rac1 activation and p47phox translocation to reduce NOX2 activation and ROX production, thus reducing cardiomyocyte apoptosis. The knockdown of SGLT1 also promoted mitochondrial fusion, inhibited mitochondrial fission, and increased ATP content and complex I–IV activity, therefore improving mitochondrial dysfunction and attenuating cardiomyocyte oxidative stress and apoptosis. Inhibition of SGLT1 can reduce cardiomyocyte hypertrophy and oxidative stress caused by intracellular Ca2+ overload, and partially decrease the inflammatory response and pyroptosis in cardiomyocytes by suppressing NF-κB and NLRP3/caspase-1 pathway.
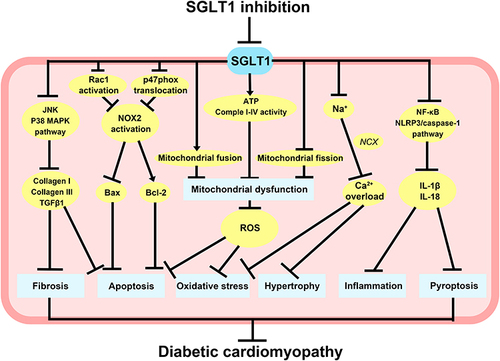
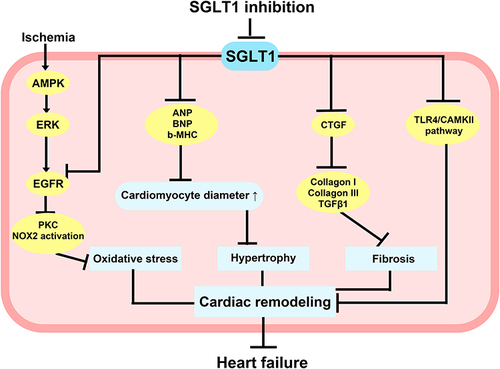
Figure 4 Potential molecular mechanisms of SGLT1 inhibition in non-diabetes-related heart injury. AMPK upregulated SGLT1 expression via the ERK pathway under ischemic conditions, and inhibition of SGLT1 attenuated the interaction of SGLT1 with EGFR, which in turn reduced PKC and NOX2 activity and alleviated oxidative stress in cardiomyocytes. Inhibition of SGLT1 reduced ANP, BNP, b-MHC, and CTGF, which contribute to preventing myocardial hypertrophy and fibrosis. Inhibition of SGLT1 also improved abnormal Ca2+ metabolism in cardiomyocytes, attenuated cardiac remodeling due to excessive activation of TLR4/CaMKII pathway, and eventually significantly reduced the incidence of heart failure.