Figures & data
Table 1 New scoring system for disseminated intravascular coagulation (DIC) by the Japanese Association for Acute MedicineCitation19
Table 2 Characteristics of systemic inflammatory response syndrome (SIRS) patients at the time of acute lung injury (ALI) diagnosis
Table 3 Types of infections and causative pathogens in acute lung injury patients with sepsis
Figure 1 Clinical efficacy of sivelestat for acute lung injury patients with systemic inflammatory response syndrome. (A) Kaplan–Meier curves for acute lung injury patients with systemic inflammatory response syndrome who did or did not receive sivelestat. Statistical analysis was performed using the log-rank test. Solid line, sivelestat patients; dashed line, control patients. (B and C) Clinical efficacy of sivelestat based on ventilator-free days (VFDs) and changes in PaO2/FIO2 (ΔP/F) before and 7 days after diagnosis of acute lung injury.
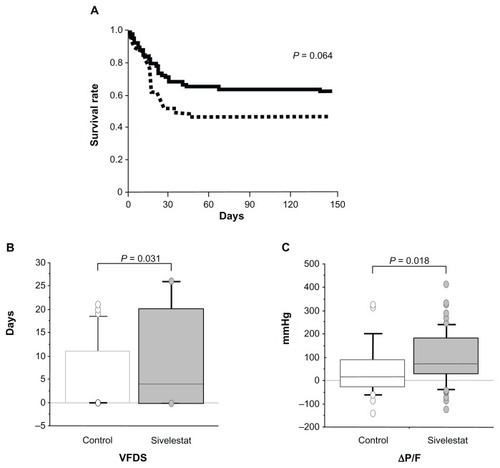
Figure 2 Hazard ratios and 95% confidence intervals for survival based on the Cox proportional hazards model for assessing acute lung injury patients with systemic inflammatory response syndrome.
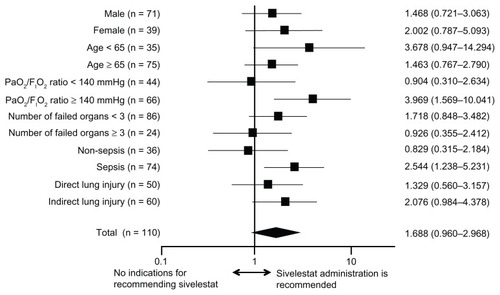
Table 4 Clinical characteristics of non-septic patients and septic patients
Figure 3 Clinical efficacy of sivelestat based on ventilator-free days (VFDs) and changes in PaO2/FIO2 (ΔP/F) before and 7 days after sivelestat administration for acute lung injury patients with systemic inflammatory response syndrome who were non-septic, septic, negative for procalcitonin (PCT), and positive for PCT. (A and C) VFD for patients who were non-septic, septic, negative for PCT, and positive for PCT. (B and D) ΔP/F for patients who were non-septic, septic, negative for PCT, and positive for PCT.
Abbreviations: Ctl, control patients; Si, sivelestat patients.
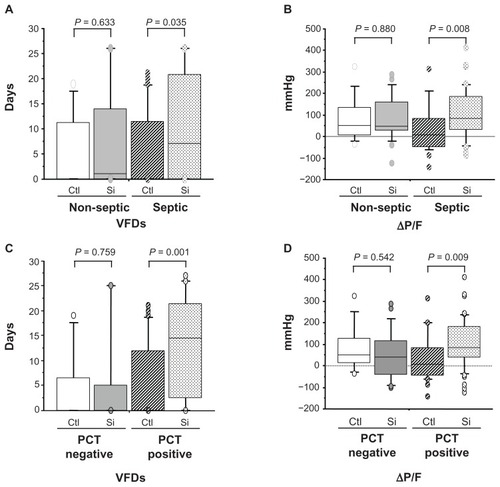
Table 5 The hazard ratios and 95% confidence intervals (CIs) for mortality based on univariate and multivariate Cox analysis in septic acute lung injury patients
Table 6 The hazard ratios and 95% confidence intervals for mortality based on univariate and multivariate Cox analysis in non-septic acute lung injury patients
Figure 4 Kaplan–Meier curves for acute lung injury patients with systemic inflammatory response syndrome who were septic (A), non-septic (B), positive for procalcitonin (PCT) (C), and negative for PCT (D).
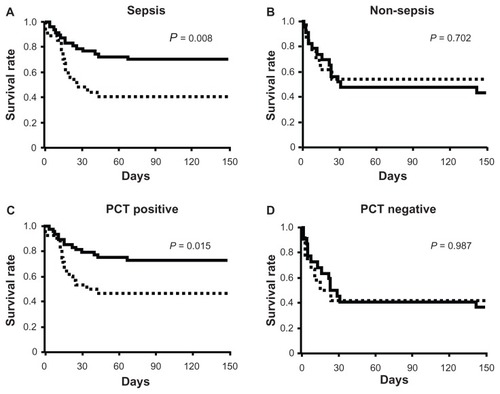
Table 7 Clinical characteristics of procalcitonin (PCT)-negative patients and PCT-positive patients
Table 8 The hazard ratios and 95% confidence intervals (CIs) for mortality based on univariate and multivariate Cox analysis in procalcitonin-positive patients