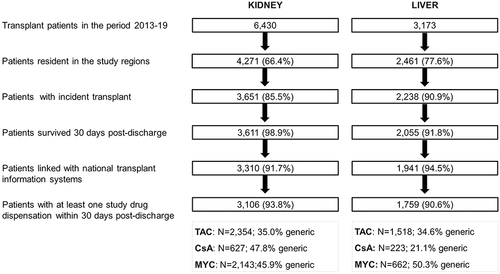
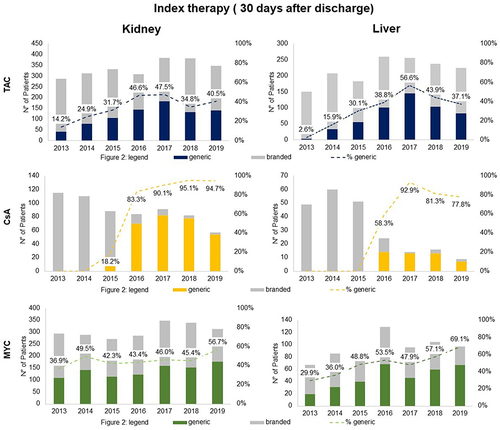
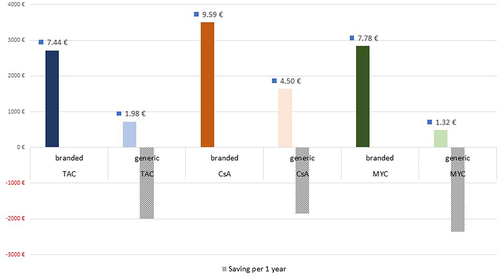
Figures & data
Table 1 Branded and Generic Version of Tacrolimus, Cyclosporine and Mycophenolate
Table 2 Transplant, Recipient and Donor Characteristics According to Generic/Branded Version by Active Ingredient in Kidney and Liver Transplantation
Table 3 Proportion of Patients Treated with Generic Version for TAC, CsA and MYC Over Time by Region in Kidney and Liver Transplantation
Figure 3 (A-C) Hospital variability in patients treated with generic drugs by active ingredient in kidney and liver transplantation.
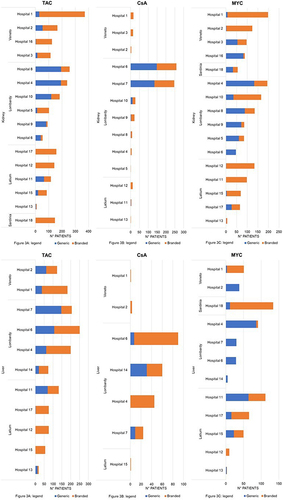
Figure 4 Proportion of patients changing drug version within one year from starting therapy by active ingredient in kidney and liver transplantation.
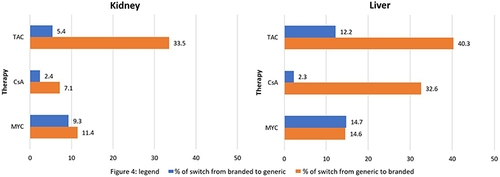
Figure 5 Proportion of patients changing drug version within two years from starting therapy by active in kidney and liver transplantation.
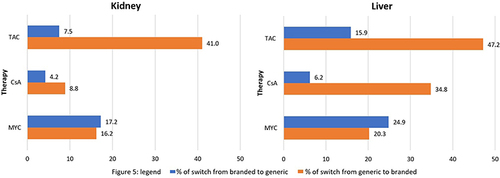
Figure 6 Risk-benefit profile of generic versus branded by active ingredient in kidney and liver transplantation.
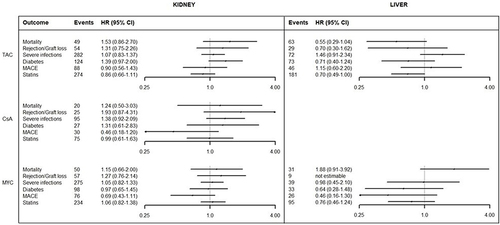
Table 4 Sensitivity Analysis Adjusting for Immunosuppressive Therapies Used in Combination with the Exposure