Figures & data
Table 1 Summary of current clinical trials of enzalutamide against PCa
Figure 2 Kaplan–Meier estimates of primary and secondary outcome measures in Phase I–II clinical trial of enzalutamide (ClinicalTrials.gov identifier: NCT00510718). (A) PSA progression defined as ≥25% increase in PSA from baseline. (B) PSA progression defined by Prostate Cancer Working Group criteria as ≥25% increase from nadir. (C) Progression-free survival defined by radiological imaging in chemotherapy-naïve and chemotherapy-treated patients. Reprinted from Scher HI, Beer TM, Higano CS, et al, Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 375:1437–1446. Copyright 2010, with permission from Elsevier.Citation49
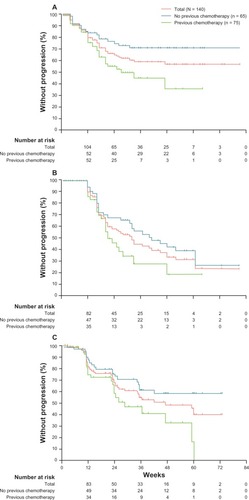
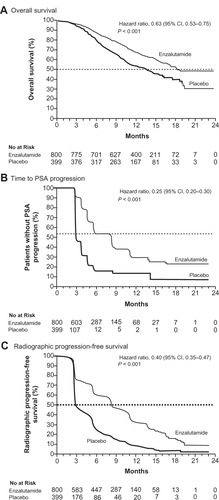
Figure 3 Kaplan–Meier estimates of primary and secondary outcome measures in Phase III clinical trial of enzalutamide (ClinicalTrials.gov identifier: NCT00974311). (A) Overall survival. (B) Progression-free survival defined by prostate-specific antigen progression. (C) Progression-free survival defined by radiological imaging. From N Engl J Med, Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. 367;1187–1197. Copyright © 2012 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.Citation52