Figures & data
Figure 1 Comparative analysis of the cytotoxic effect of various metallo-salophenes on platinum resistant ovarian cancer cell lines. (A) Structure of salophene ligand (SP) and metal-salophene complex (Me-SP). (B) Human ovarian cancer cells (SKOV-3, OVCAR-3) were treated for 24 h with various concentrations (0.1–60 μM) of either newly synthesized transition metal salophene complexes (Fe(III)-SP, Co(III)-SP, Mn(II)-SP, and Cu(II)-SP) or the respective metal chloride alone (Fe3+, Co3+, Mn2+, Cu2+) or the noncomplexed parent compound (SP). The MTS viability assay was carried out as described (Materials and methods). Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X ± SD) of a representative experiment in % cell viability of untreated cells (100%).
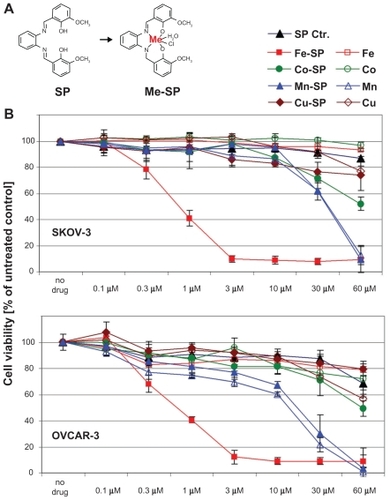
Figure 2 Differential effect of Fe-SP on the viability of various human cancer cells. The cytotoxic effect of Fe-SP (0–3 μM) on human ovarian cancer cells (SKOV-3, OVCAR-3, CaOV-3) was compared to various human cancer cell lines of different origin (LNCaP, PC-3, BxPC-3, A-431, SH-SY5Y). Treatment with 3 μM SP served as a negative control. The MTS viability assay was carried out as described (Materials and methods).
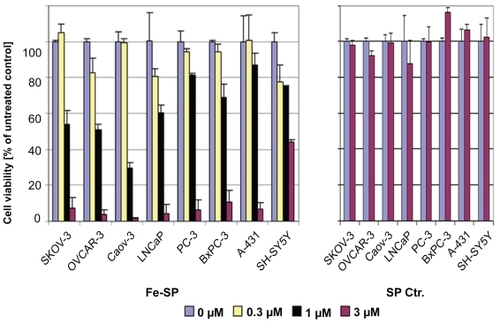
Figure 3 Kinetics of the cytotoxic effect of Fe-SP on SKOV-3 ovarian cancer cells. SKOV-3 cells were treated with various concentrations (0–10 μM) of Fe-SP for 3, 6, 12, 24, or 48 h. The MTS viability assay was carried out as described (Materials and methods).
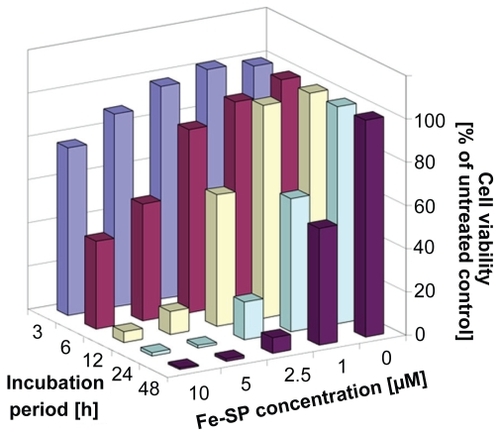
Figure 4 Determination of mitochondrial membrane depolarization, apoptotic and necrotic SKOV-3 cells after Fe-SP treatment. (A) Mitochondrial membrane depolarization analysis. SKOV-3 cells were treated for 3 h with 3 μM Fe-SP or SP control, fixed and stained with DiOC18(3) as described (Materials and methods). Fluorescence of the single cell population was measured by flow cytometry (right panel) and the transmembrane depolarization potential of the single cell populations plotted (bar chart, left panel). Ten thousand cells were analyzed in each sample. (B) Apoptotic and necrotic cell population. SKOV-3 cells were treated with 1 μM Fe-SP or SP control for 24 h and floating and attached cells collected and combined. The quantification of apoptotic cells (Annexin V plasma membrane staining) and necrotic cells (7-AAD DNA staining) of SKOV-3 cells was carried out by flow cytometry as described (see Materials and methods). Ten thousand events were analyzed for each sample.
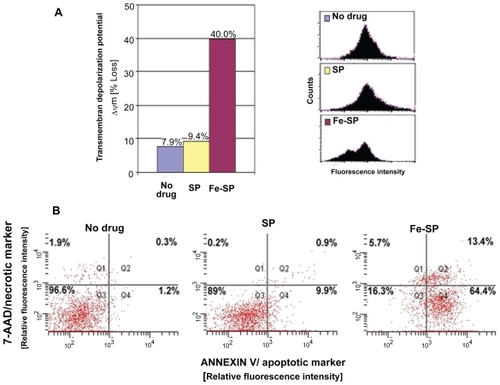
Table 1 Acute toxicity of iron salophene
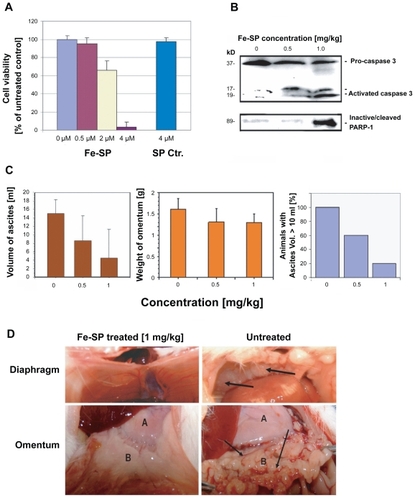
Figure 5 Chemotherapeutic effect of Fe-SP in an animal ovarian cancer cell model. (A) Effect of Fe-SP on the viability of rat ovarian cancer cells (NUTU-19) in vitro. Rat ovarian cancer cells (NUTU-19) were plated into 96 well flat bottom plates and treated with various concentrations (0.5–4 μM) of Fe-SP or 4 μM noncomplexed SP. The MTS viability assay was carried out as described (Materials and Methods). (B) In vivo activation of Caspase-3 and in-activation of PARP-1 in ovarian cancer cell-derived tumors after Fe-SP treatment. NUTU-19 derived tumor tissue of nontreated or Fe-SP treated (0.5 mg/kg or 1.0 mg/kg bodyweight) rats was pooled for each treatment group. Cell lysates were prepared in the presence of a broad range of proteinase inhibitors to prevent protein degradation and the expression of caspase-3 and PARP-1 was analyzed by immunoblotting (see Material and methods). Cell lysates 50 μg of total cellular protein/lane were separated on a 12% SDS-polyacrylamide gel. PARP-1 was visualized using a primary antibody solely recognizing the inactivated/cleaved protein. For caspase-3 an antibody which recognizes the full length pro-form as well as activated/cleaved fragments of the protein was used. Thus, a direct conversion of the precursor into activated caspase-3 was monitored for all samples allowing direct comparison of loading between samples from treated and nontreated animals. (C) Volume of ascites and weight of omentum from Fe-SP treated rats with SKOV-3 derived tumors. Graphs show a trend for decreased ascites and omental tumor burden after Fe-SP (0.5, 1.0 mg/kg) treatment. (D) Image of diaphragm and omentum. Example of complete response of a SKOV-3 derived tumor to Fe-SP at concentration of 1 mg/kg body weight. Following treatment both the diaphragm and omentum are disease free.