Figures & data
Table 1 The Passive Targeting and Functional Effects of Nanoformulations for IBD Treatment
Table 2 The Active Targeting and Functional Effects of Nanoformulations for IBD Treatment
Table 3 The Hybrid Targeting and Functional Effects of Nanoformulations for IBD Treatment
Table 4 The Effectiveness and Limitations of Different Types of Drug Delivery Systems for the Treatment of IBD
Figure 1 Strategies for inflammatory bowel disease treatment using nanoparticle-based drug delivery systems. Nanoparticles specifically target inflammatory colonic epithelial cells based on enhanced permeability and retention effects (A), specific enzyme levels (B), reactive oxygen species (ROS) levels (C), specific pH levels (D), electrostatic interactions (E), and ligand-receptor interactions (F).
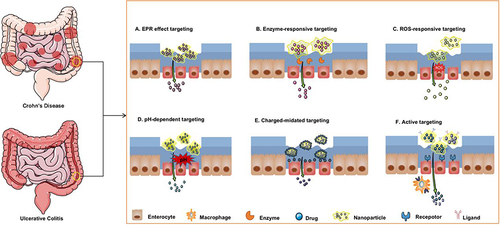
Figure 2 The expression of myeloperoxidase activity (A), TNF-α (B), and IL-1β (C) was significantly decreased in the colon of mice treated with budesonide-nanostructured lipid carriers compared to the control group. Reprinted from Int J Pharm, volume 454(2), Beloqui A, Coco R, Alhouayek M, et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. 775–783, Copyright 2013, with permission from Elsevier.Citation26
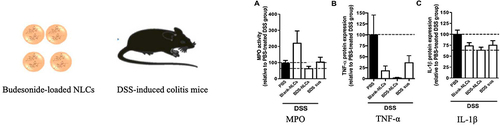
Figure 3 Lysozyme-triggered release of vancomycin from chitosan microgels for treating inflammatory bowel disease. (A) Schematic representation and mechanism of action of lysozyme-triggered nanoparticles. (B) Determination of Caco-2 cell activity in various treatment groups. (C) Inhibitory effect of N-CH-PANI@VM MGs on Staphylococcus aureus in various environments. (D) Cumulative release of lysozyme-induced VM in various simulated environments. Adapted from J Adv Res, volume 43, Li X, Hetjens L, Wolter N, et al. Charge-reversible and biodegradable chitosan-based microgels for lysozyme-triggered release of vancomycin. 87–96, Copyright 2023, with permission from Elsevier.Citation35
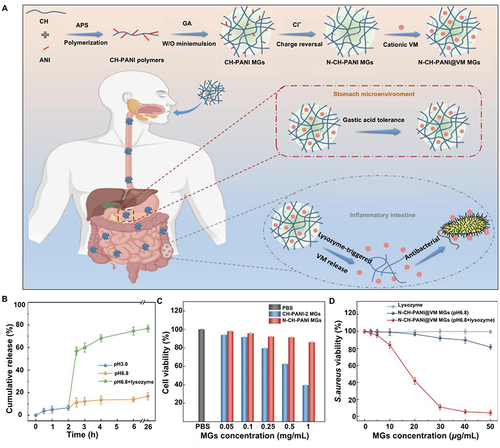
Figure 4 Sensitive reactive oxygen species-responsive B-ATK-T nanoparticles (NP) for treating irritable bowel disease. (A) The synthetic process of B-ATK-T. (B) Hydrolysis rate of B-ATK-T NP at various concentrations of hydrogen peroxide (H2O2). The release profiles of 9-fluorenone (C), budesonide (D), and tempol (E) from B-ATK-T NP at varying concentrations of H2O2 concentrations. Reprinted from J Control Release, volume 316, Li S, Xie A, Li H, et al. A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. 66–78, copyright 2019, with permission from Elsevier.Citation43
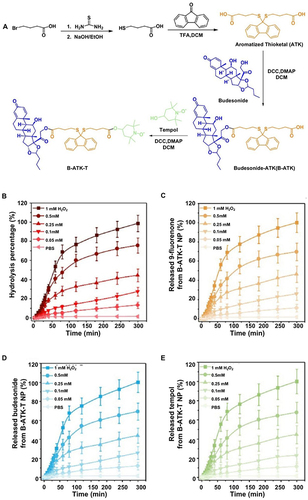
Figure 5 The novel nano-delivery system MNEHDS for treating irritable bowel disease. (A) The manufacturing process of MNEHDS. (B) Berberine (BBR) drug release rates in various simulated environments. The changes in BBR concentrations were investigated at various intervals in the plasma (C), colon (D), and feces (E). Reprinted from Zhang L, Li M, Zhang G, et al. Micro- and nanoencapsulated hybrid delivery system (MNEHDS): a novel approach for colon-targeted oral delivery of berberine. Mol Pharmaceut. 2021;18(4):1573–1581. Copyright © 2021 American Chemical Society.Citation49
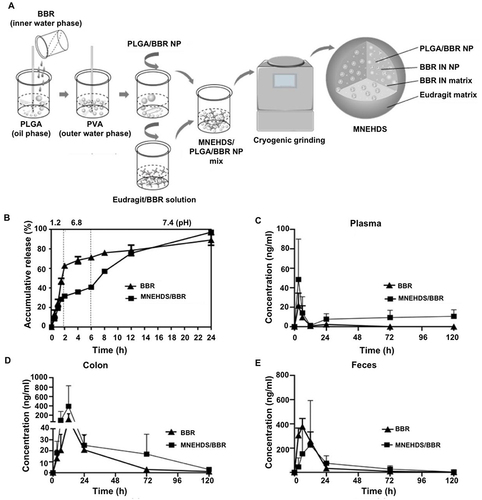
Figure 6 AceKGM nanoparticles for the treatment of irritable bowel disease. The percentage change in mice from various treatment groups body weight (A), Disease Activity Index score (B), myeloperoxidase activity (C), colon length (D), and TNF-α content (E). (F) Mice colonic tissues from various treatment groups. Reprinted from Wang C, Guo Z, Liang J, et al. An oral delivery vehicle based on konjac glucomannan acetate targeting the colon for inflammatory bowel disease therapy. Front Bioeng Biotechnol. 2022;10:1025155. Creative Commons.Citation75
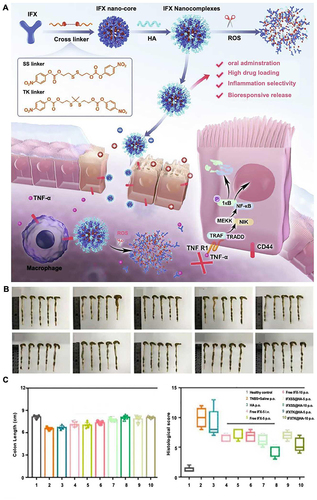
Figure 7 (A) IFXSS@HA/IFXTK@HA drug synthesis process and irritable bowel disease treatment mechanism. Colonic tissues (B), colonic length, and histopathologic histologic scores (C) of mice post-treatment in each group. Adapted from Chem Eng J, volume 445, Li X, Fang S, Yu Y, et al. Oral administration of inflammatory microenvironment-responsive carrier-free infliximab nanocomplex for the targeted treatment of inflammatory bowel disease. 136438, Copyright 2022, with permission from Elsevier.Citation97