Figures & data
Figure 1 Composition of the endocannabinoid system. The endocannabinoid system consists of CB1R, CB2R, endocannabinoids, and their corresponding synthesizing and degrading enzymes. 2-AG and AEA, the primary endocannabinoids, are produced on demand and are synthesized from the postsynaptic terminals by DAGLα and NAPE-PLD, respectively, to activate presynaptic cannabinoid receptors. CB1R activation inhibits presynaptic neurotransmitter release and promotes astrocytic glutamate release. CB2R activation reduces microglial inflammatory factor production. 2-AG and AEA are enzymatically degraded to AA by MAGL and FAAH hydrolases, and can also be oxidatively degraded to PG-EA and PG-G by COX-2.
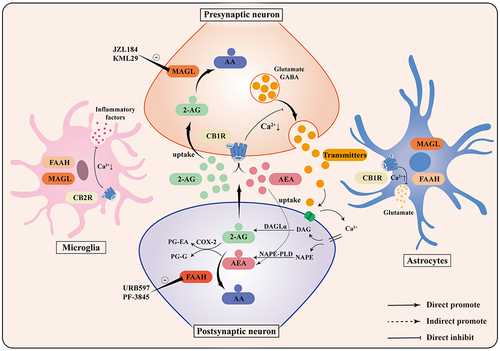
Table 1 Summary of the Anxiolytic Effects of MAGL Inhibitors in Preclinical Studies
Figure 2 Schematic representation of the mechanism of anxiolytic action mediated by MAGL inhibitors. Briefly, the anxiolytic effects of MAGL are related to its maintenance of Glutamate/GABA balance, inhibition of neuroinflammation, and regulation of corticosterone levels.
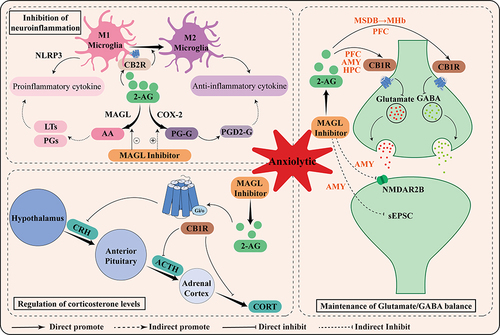
Table 2 Summary of MAGL Inhibitors Derived from Natural Products
Table 3 Summary of the Anxiolytic Effects of FAAH Inhibitors in Preclinical Studies
Figure 3 Schematic representation of the mechanism of anxiolytic action mediated by FAAH inhibitors. Briefly, the anxiolytic effects of FAAH are related to its maintenance of Glutamate/GABA balance, suppression of neuroinflammation, regulation of HPA axis, and promotion of neurogenesis and plasticity.
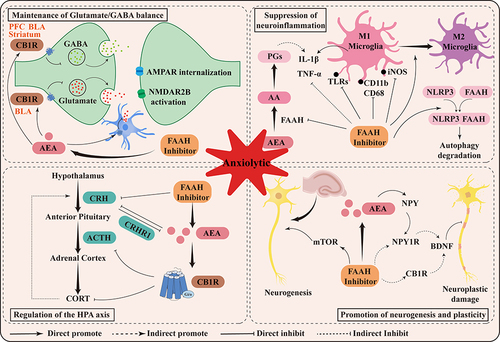
Table 4 Summary of FAAH Inhibitors Derived from Natural Products
