Figures & data
Figure 1 Study design.
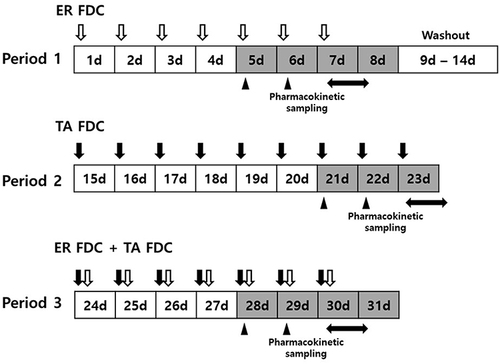
Table 1 Pharmacokinetic Parameters of Total Ezetimibe, Free Ezetimibe, and Rosuvastatin After Multiple Administrations of the Fixed-Dose Combination (FDC) of Ezetimibe (20 Mg) and Rosuvastatin (10 Mg), Both Alone and in Combination with the FDC of Telmisartan (80 Mg) and Amlodipine (5 Mg)
Figure 2 Mean plasma concentration‒time profiles of the lipid-lowering agents (A) total ezetimibe, (B) free ezetimibe, and (C) rosuvastatin at steady state after multiple administrations of ER-FDC alone and with TA-FDC.
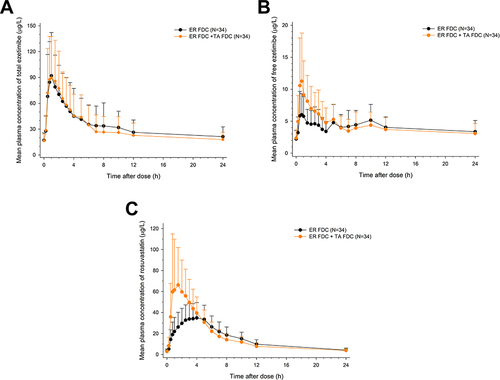
Figure 3 Comparison of Cmax,ss and AUCtau,ss for lipid-lowering agents, total ezetimibe, free ezetimibe, and rosuvastatin after multiple administrations of ER FDC alone and with TA FDC.
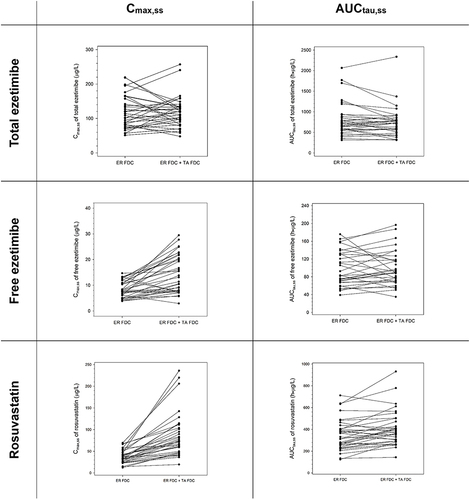
Table 2 Pharmacokinetic Parameters of Telmisartan and Amlodipine After Multiple Administrations of the Fixed-Dose Combination (FDC) of Telmisartan 80 Mg and Amlodipine 5 Mg FDC, Both Alone and in Combination with Ezetimibe 20 Mg and Rosuvastatin 10 Mg
Figure 4 Mean plasma concentration‒time profiles of antihypertensive agents, (A) telmisartan, and (B) amlodipine at steady state after multiple administrations of TA FDC alone and with ER FDC.
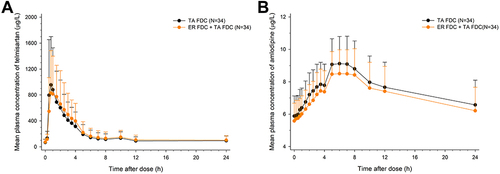
Figure 5 Comparison of Cmax,ss and AUCtau,ss for antihypertensive agents, telmisartan, and amlodipine after multiple administrations of TA FDC alone and with ER FDC.
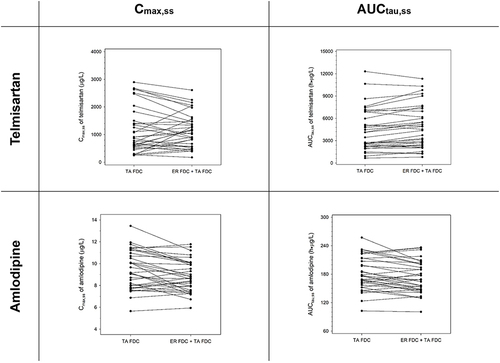
Table 3 Summary of Adverse Drug Reactions
Data Sharing Statement
The data supporting the published results of this study will be shared upon a reasonable request made to the corresponding author or sponsor.
