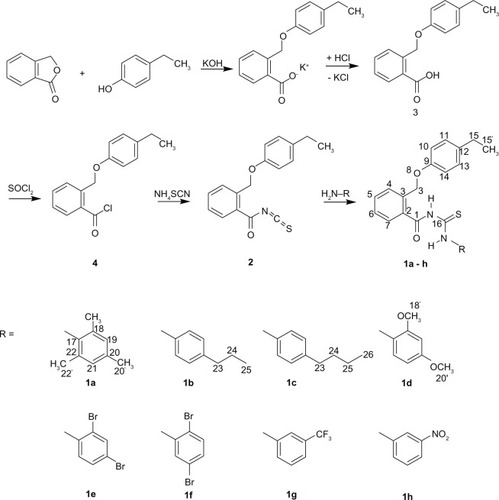
Figures & data
Figure 2 Percent inhibition of carrageenan paw edema for N-phenylcarbamothioylbenzamides 1a–h in mice.a
aDoses administered: indomethacin and test compounds 1a–h (0.028 mmol). Percentage of inhibition of edema expressed as means of five replicates ± standard error of the mean. *Significantly statistically different from IND group (P = 0.049 for 1a, 0.0015 for 1d, and 0.033 for 1f); **significantly statistically different from IND group and groups treated with 1a (P = 0.0014 for 1e and 0.0022 for 1h), 1b, 1c, and 1d (P = 0.031 for 1e), 1f (P = 0.026 for 1e), and 1g (P = 0.0011 for 1h); #no activity observed.
Abbreviations: CON, placebo control; IND, positive control.
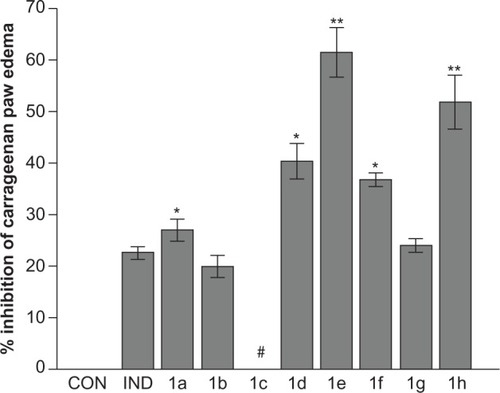
Table 1 Effect of indomethacin and compounds 1a, 1e, and 1h on ulcerogenic properties
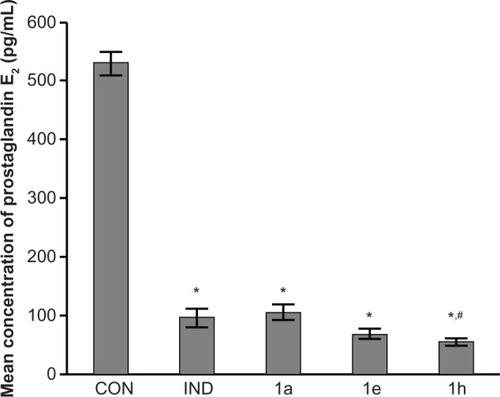
Figure 3 Inhibition of prostaglandin E2 (PGE2) by test compounds 1a, 1e, and 1h.
Abbreviations: CON, placebo control; IND, positive control.