Figures & data
Table 1 The validation data of the analytical method used to determine candesartan in human plasma by UPLC-MS/MS using irbesartan as the internal standard
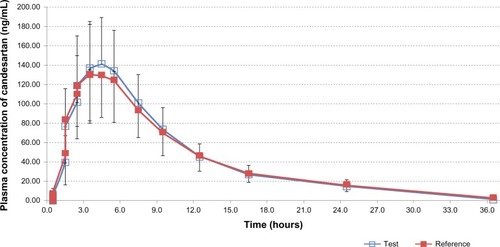
Table 2 Pharmacokinetic parameters and statistical comparison of candesartan after single-dose oral administration of a 16 mg candesartan cilexetil tablet of the test and the reference drug