Figures & data
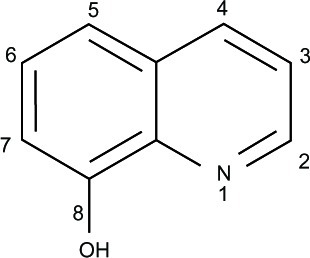
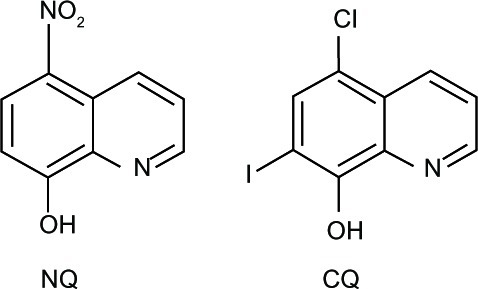
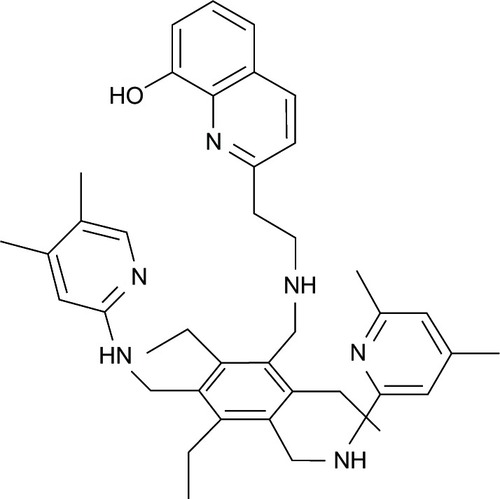
Figure 2 8-Hydroxyquinoline derivatives with potent antineurodegenerative activity.
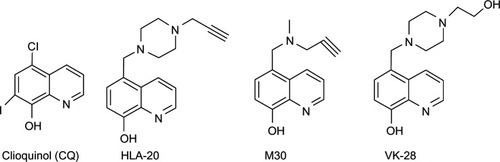
Figure 4 M30 and HLA-20 are hybrids of Ladostigil and VK-28.
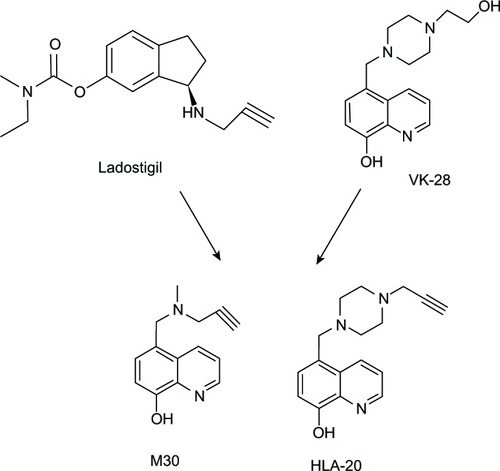
Figure 6 Metabolism of dopamine and actions of 8-hydroxyquinoline derivatives.
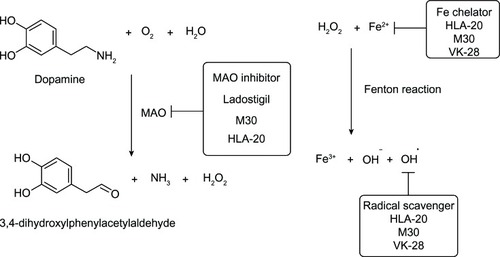
Figure 9 Glucoconjugates of 8-hydroxyquinoline and clioquinol.
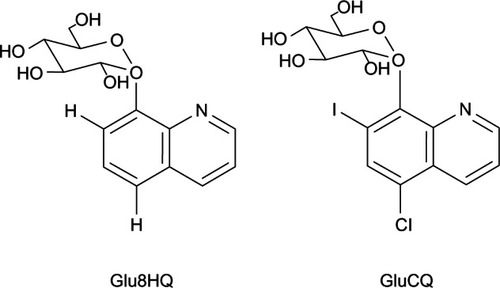
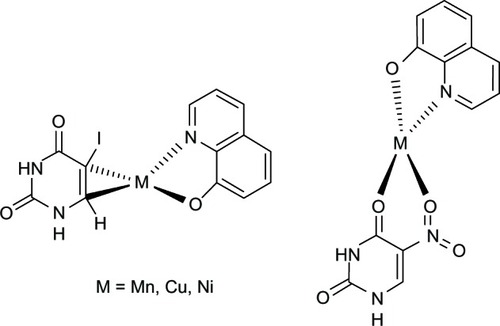
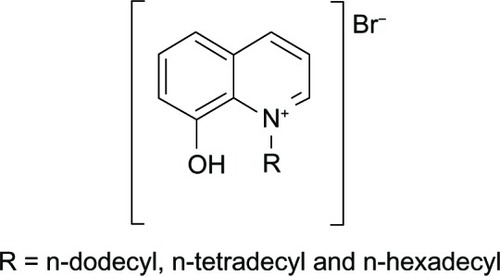
Figure 10 Chemical structures of HQMABS and metal complexes.
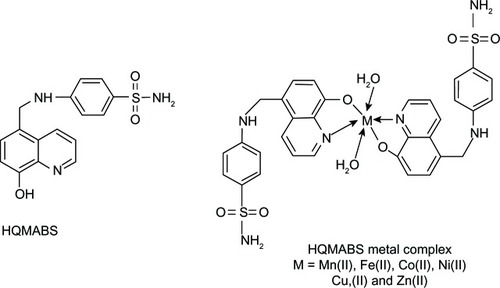
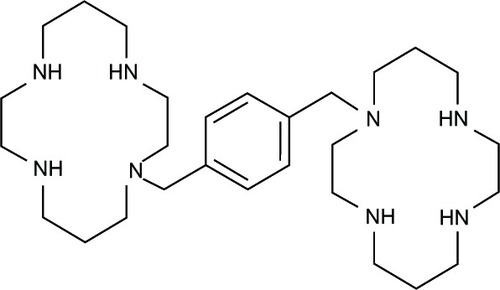
Figure 13 Chemical structures of hydroxyquinoline-polyamine conjugates using hydroxyquinoline conjugation with polyamine backbone or polyazamacrocycles.
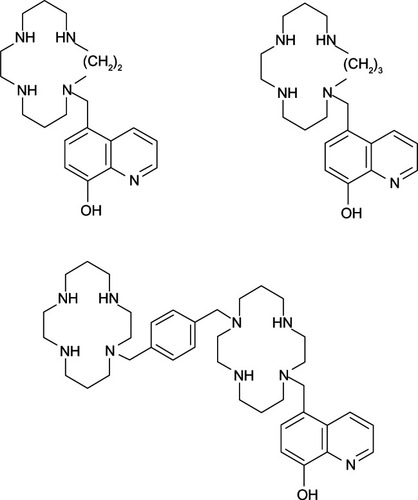
Figure 17 FOXO1 functions are related to glucose homeostasis and providing protection against oxidative stress.
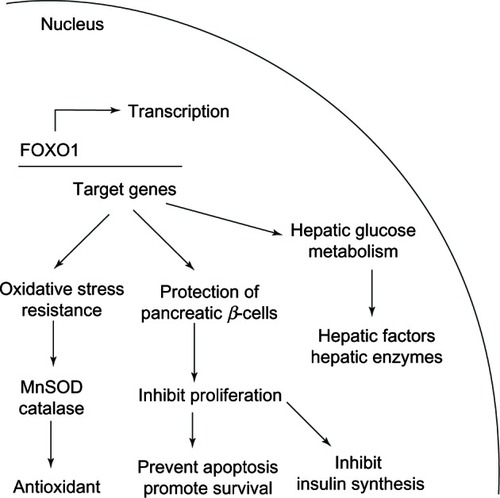
Figure 18 The role of CQ as Zn ionophore in controlling blood glucose level via inhibition of FOXO1.
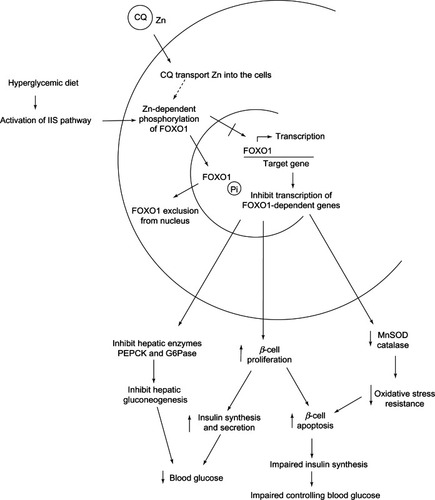
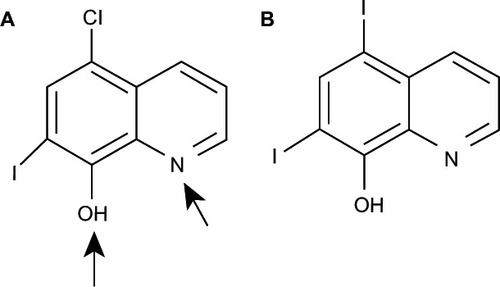
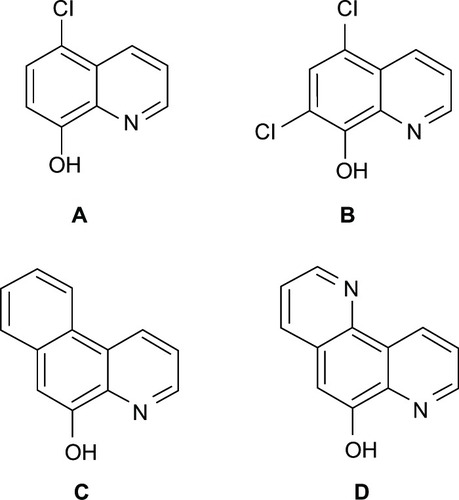
Figure 19 8HQ derivatives with different substitutions.
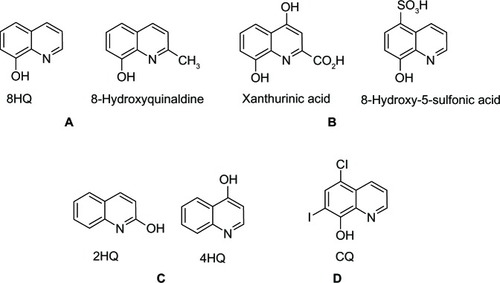
Figure 20 Liver functions on controlling glucose metabolism and antidiabetic actions of 8-hydroxyquinoline derivatives.
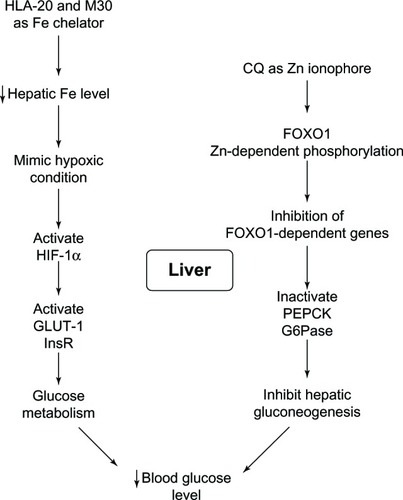
Table 1 Brief review of 8-hydroxyquinoline (8HQ) and its derivatives
Table 2 Bioactivities and specific mechanisms of 8-hydroxyquinoline (8HQ) and its derivatives