Figures & data
Figure 1 Pre-experimental design.
Abbreviations: M, month; D, day; CNI, calcineurin inhibitor.
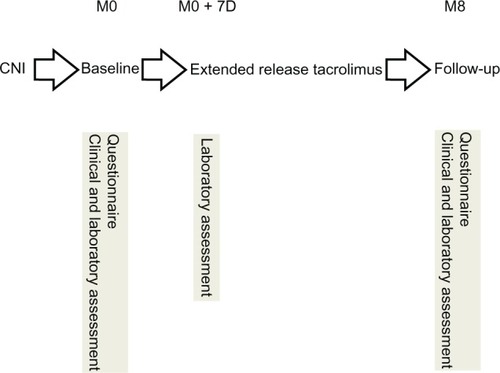
Table 1 Patient characteristics (n=76)
Figure 2 Overall self-reported adherence at baseline and 8 months after conversion to modified-release tacrolimus (BAASIS).
Abbreviation: Baasis, Basel assessment of adherence with immunosuppressive Medication scale.
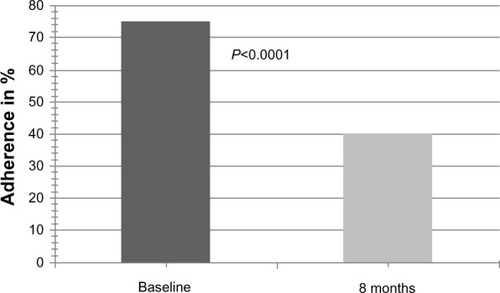
Figure 3 Overall self-reported adherence at baseline and 8 months after conversion to modified-release tacrolimus (BAASIS-VAS scale).
Abbreviations: Baasis, Basel Assessment of Adherence with Immunosuppressive Medication Scale; VAS, visual analog scale.
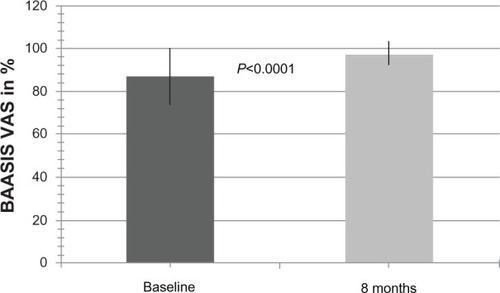
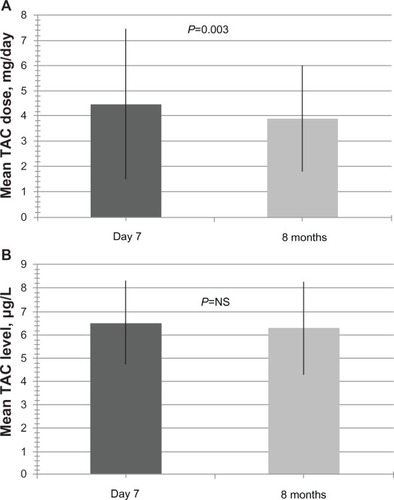
Figure 4 Modified-release tacrolimus doses and levels at day 7 and month 8 after conversion to modified-release tacrolimus.
Abbreviation: TAC, tacrolimus.