Figures & data
Table 1 Composition of gastroretentive formulations of acyclovir
Figure 1 Comparison of simulated steady state plasma concentration profiles of the IR and GR formulations of acyclovir along with target drug release profile.
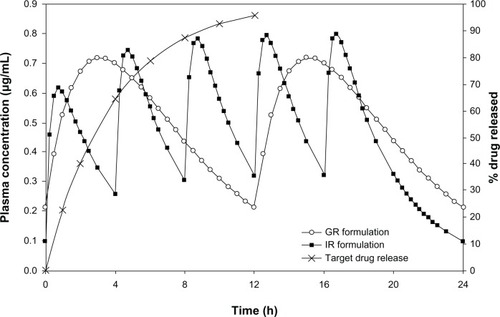
Table 2 Results of drug–excipient compatibility study of acyclovir
Table 3 Characterization of acyclovir
Table 4 In-process characterization of different batches of acyclovir gastroretentive tablets
Figure 2 In vitro drug release profiles of gastroretentive tablets of acyclovir.
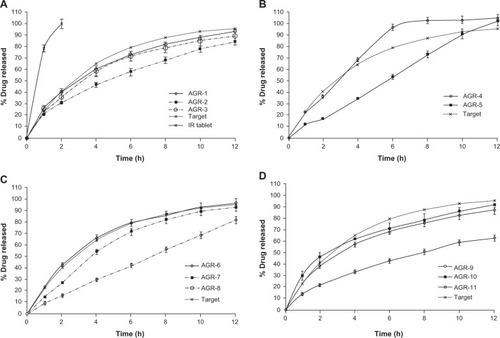
Table 5 Similarity factor and splitting behavior of acyclovir gas-troretentive tablets
Table 6 Mathematical modeling and drug-release kinetics of acyclovir gastroretentive formulations
Figure 3 Swelling index of gastroretentive tablets of acyclovir.
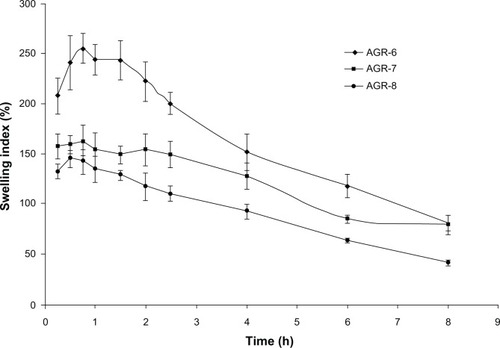
Figure 4 Matrix erosion of gastroretentive tablets of acyclovir.
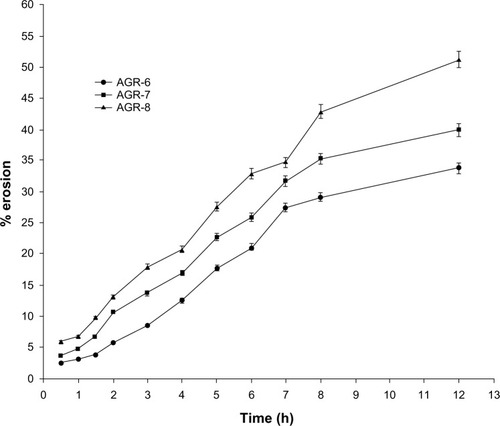
Figure 5 Relative size of optimized GR formulation.
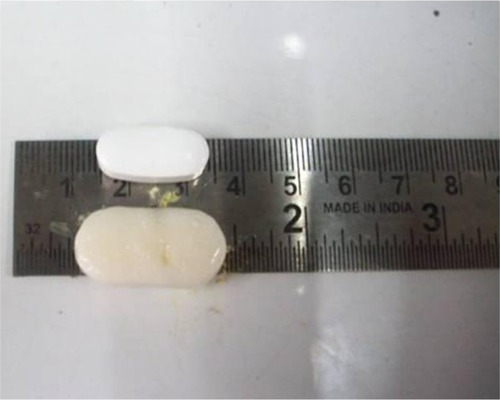
Figure 6 Detachment forces of different batches of gastroretentive and conventional acyclovir tablets in mucoadhesion study.
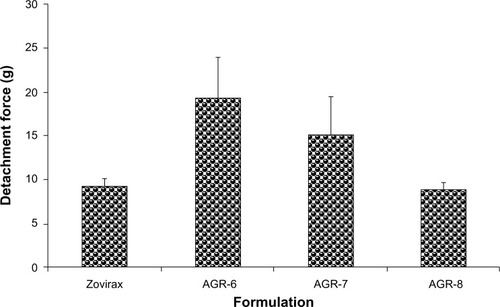
Figure 7 X-ray radiography photographs of rabbit administered with IR hours (A) and 8 hours (B) with optimized GR formulation (AGR-6).
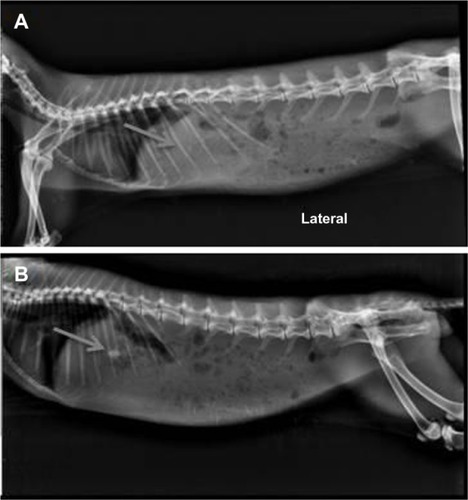
Table 7 Pharmacokinetic parameters of acyclovir after administration of GR and IR formulations
Figure 8 Mean plasma concentration profiles of IR and optimized GR formulation (AGR-6).
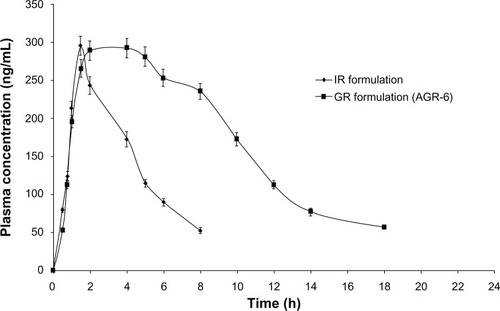
Table 8 Stability data of optimized batch of acyclovir gastro-retentive sustained-release formulation (AGR-6)