Figures & data
Table 1 Indications for zoledronic acid based on selected published studies
Figure 1 Incidence of fractures during the 3-year study period.Citation7 The primary end points – the incidence of morphometric vertebral fracture (Panel A) and the 3-year incidence of hip fracture (Panel B) – are shown for both groups. In Panel A, the 5675 patients in stratum 1 who were included in the analysis underwent radiography at baseline and at least once during follow up. Any missing data for earlier visits were imputed from later visits, and missing data for later visits were imputed from earlier visits. The total number of follow up radiographs were 5675 at 1 year, 5308 at 2 years, and 4969 at 3 years. Secondary end points – nonvertebral fracture (Panel C), any clinical fracture (Panel D), and clinical vertebral fracture (Panel E) – are also shown over a 3-year period. In panels B, C, D, and E, the number of subjects at 36 months is the number who had closeout visits on or after the start of the 36-month window for visits.
Reproduced with permission from Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.Citation25 © 2007 Massachusetts Medical Society. All Rights Reserved.
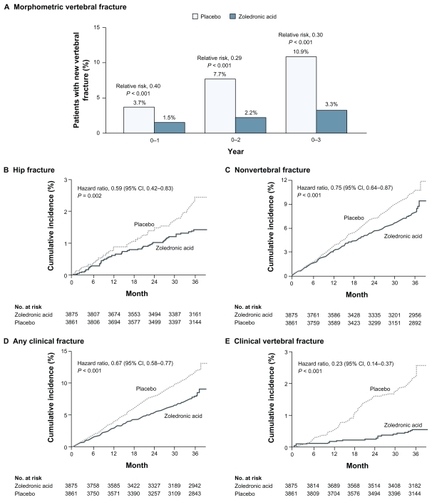
Table 2 Indications for zoledronic acid, based on selected as yet unpublished studies
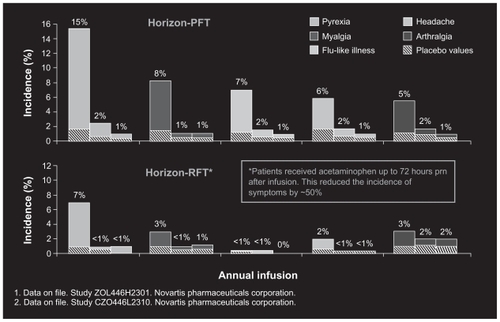
Figure 2 Most common adverse events within 3 days after infusion in HORZON PFT and RFT. (Unpublished data. Study ZOL445H2301, Novartis Pharmaceuticals and Study CZOL446L2310, Novartis Pharmaceuticals).