Figures & data
Figure 1 Synthesis of N2-substituted 5-aryl-tetrazolyl alkylamines.
Abbreviations: Ar, aromatic nucleus; DMF, N, N-dimethyl formamide; aq, aqueous; rt, room temperature; EtOH, ethanol.
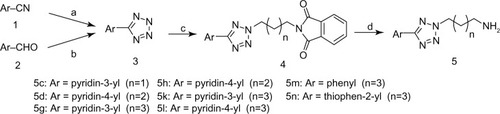
Figure 2 Synthesis of N1-submitted and N2-submitted 5-aryl-tetrazolyl alkylamines.
Abbreviations: DMF, N, N-dimethyl formamide; EtOH, ethanol.
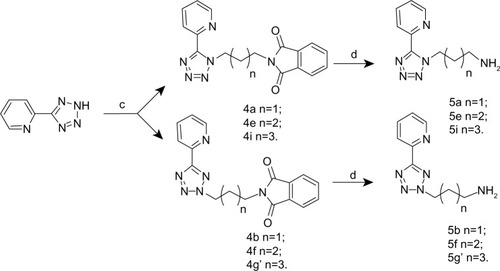
Figure 3 Synthesis of a set of acylide derivatives for compounds 10a–10g.
Abbreviations: TEA, triethylamine; PivCl, pivaloyl chloride; EtOH, ethanol; CDI, carbonyl diimidazole; DMAP, 4-Dimethylaminopyridine; Ac, acetoxy; rt, room temperature.
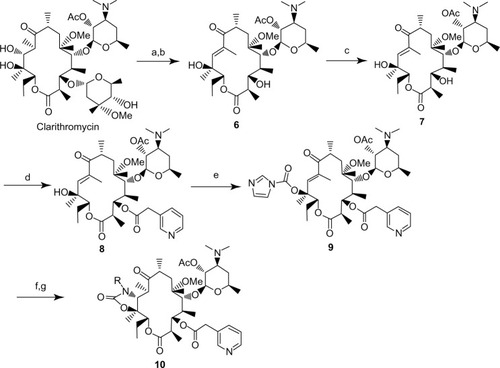
Figure 4 Structure synthesized acylides.
Abbreviations: R, substituted group; Me, methyl.
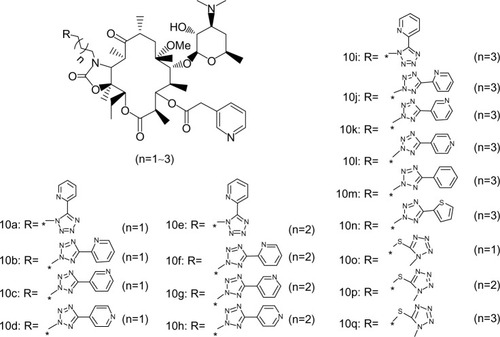
Table 1 The antibacterial activities of novel acylides in vitro
