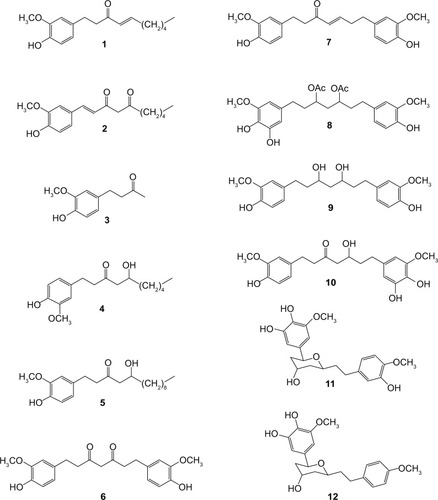
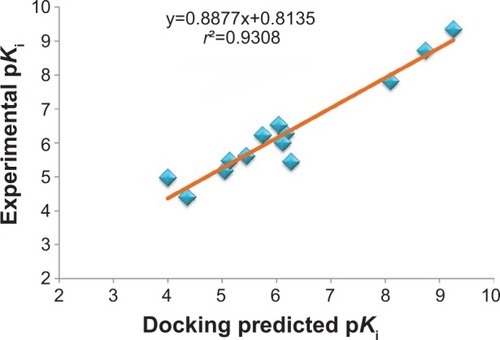
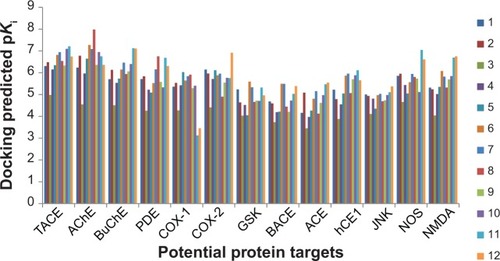
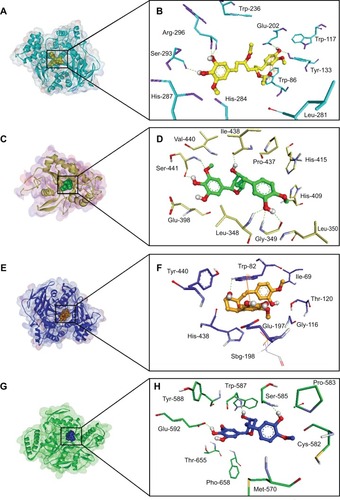
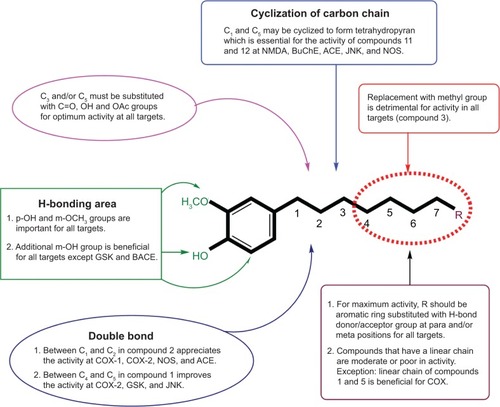
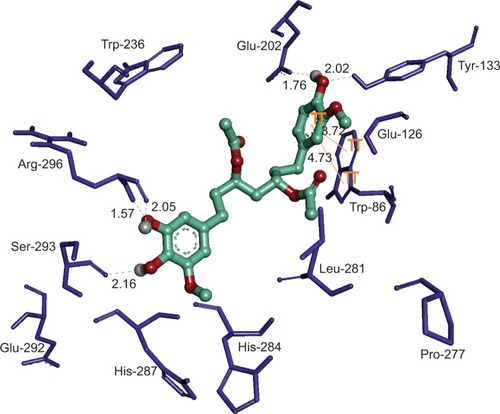
Figures & data
Table 1 Various mechanisms associated with ginger
Table 2 Protein targets with anti-Alzheimer’s effect or target enzymes of drug design selected for docking studies
Table 3 Results obtained after docking of ginger compounds (1–12) with various protein targets
Table 4 Physicochemical parameters for good oral bioavailability of ginger compounds (1–12)
Table 5 Drug-likeness/scores and toxicity calculations of ginger compounds based on Osiris property explorer