Figures & data
Figure 1 Subjects (n=30) were randomized to one of the six sequence groups (five in each sequence in period 1). Subjects were administered the study drugs with a 1-week washout between treatments.
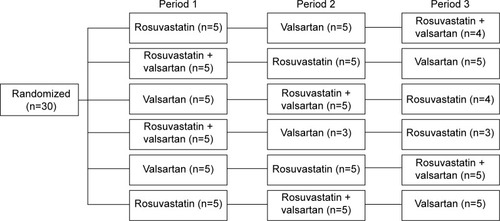
Table 1 Steady-state pharmacokinetic properties of rosuvastatin and valsartan after multiple oral administrations of rosuvastatin and valsartan alone and in combination in healthy male subjects
Table 2 Geometric mean ratios and the 90% confidence intervals (CIs) for rosuvastatin and valsartan pharmacokinetic variables when administered alone (reference) and in combination (test) in healthy male subjects
Figure 2 Plasma concentration–time profiles of rosuvastatin (A) and N-desmethyl rosuvastatin (B) after multiple oral administrations of rosuvastatin alone and coadministrations of rosuvastatin and valsartan.
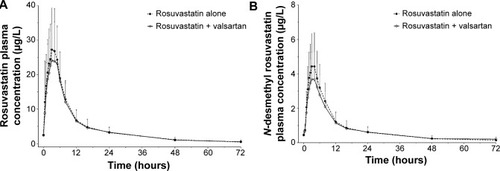
Figure 3 Plasma concentration–time profiles of valsartan after multiple oral administrations of valsartan alone and coadministrations of valsartan and rosuvastatin.
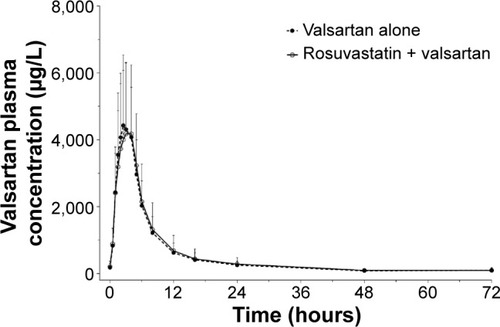
Table 3 Changes from baseline in systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate (PR) after multiple oral administrations of rosuvastatin and valsartan alone and in combination in healthy male subjects
Table 4 Change from baseline in lipid profiles following multiple oral administrations of rosuvastatin and valsartan alone and in combination in healthy male subjects
Table 5 Summary of drug-related adverse events after multiple oral administration of rosuvastatin and valsartan alone and in combination in healthy male subjects
