Figures & data
Figure 1 Photomicrographs of immunoreactivity (immunohistochemistry) and Western blots of HIF-1α, VEGF, and PECAM-1 in the cortical peri-infarct area at 14 days after IR.
Abbreviations: GSNO, S-nitrosoglutathione; IR, ischemia–reperfusion; Sham, sham-operated animals; VEGF, vascular endothelial growth factor; HIF-1α, hypoxia-inducible factor-1 alpha.
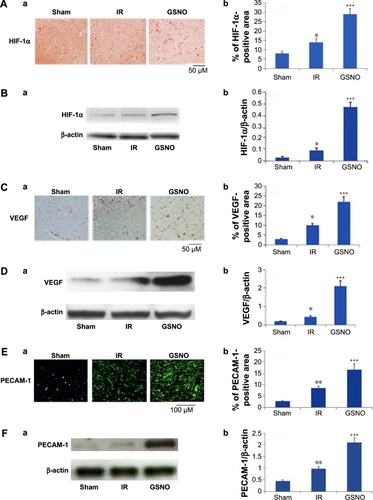
Figure 2 Photomicrographs of immunohistochemistry of blood vessel markers and cell proliferation marker at 14 days after IR.
Abbreviations: GSNO, S-nitrosoglutathione; IR, ischemia–reperfusion; Sham, sham-operated animals; GSL-1, Griffonia simplicifolia lectin 1.
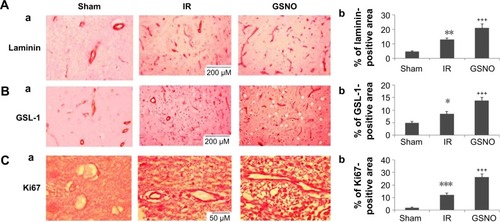
Figure 3 Effect of GSNO on improvement of neurobehavioral functions at 14 days after IR.
Abbreviations: GSNO, S-nitrosoglutathione; IR, ischemia–reperfusion; mNSS, modified neurological severity score; Sham, sham-operated animals.
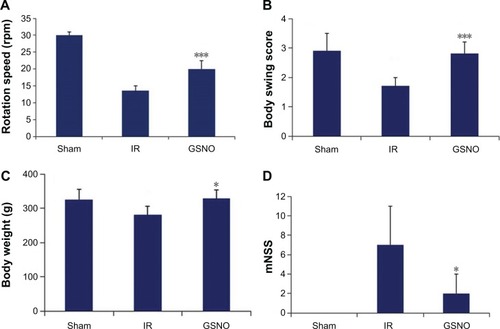
Figure 4 Effect of inhibition of HIF-1α by 2-ME on GSNO-mediated protective effects at 7 days after IR.
Abbreviations: 2-ME, 2-methoxyestradiol; GSNO, S-nitrosoglutathione; IR, ischemia–reperfusion; Sham, sham-operated animals; TTC, 2,3,5-triphenyltetrazolium chloride; HIF-1α, hypoxia-inducible factor-1 alpha.
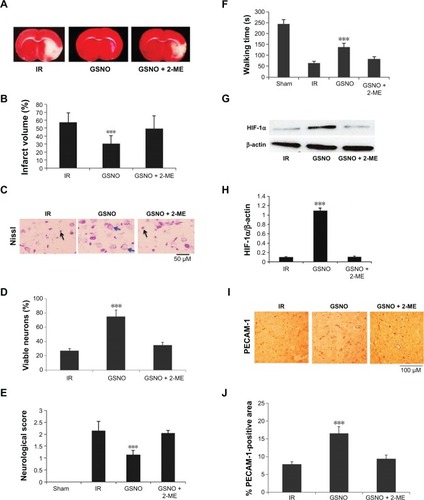
Figure 5 Effects of GSNO on expression of HIF-1α and VEGF in HIF-1α-silenced endothelial (bEnd3) cells.
Abbreviations: GSNO, S-nitrosoglutathione; Scr, scrambled; Untr, untreated; VEGF, vascular endothelial growth factor; HIF-1α, hypoxia-inducible factor-1 alpha; RIPA, radioimmunoprecipitation assay.
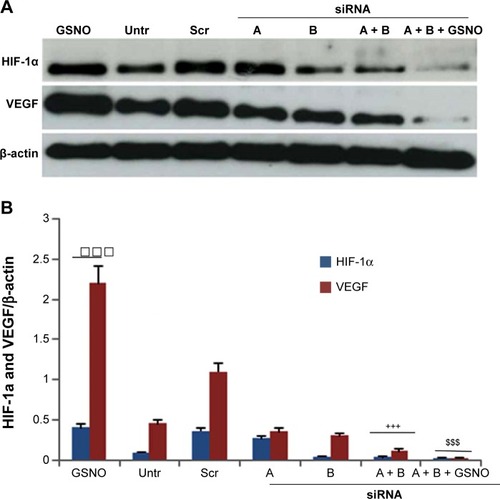
Figure 6 Effect of GSNO on formation of capillary-like proangiogenic structures on endothelial cells.
Abbreviations: 2-ME, 2-methoxyestradiol; GSNO, S-nitrosoglutathione; Untr, untreated.
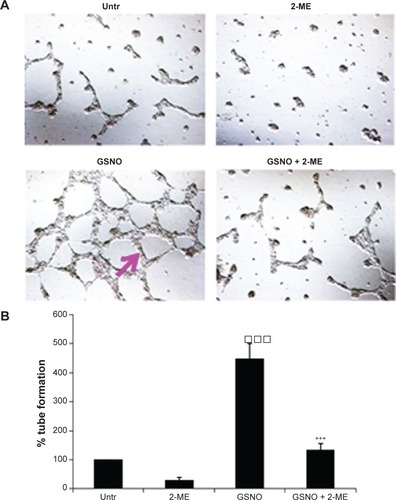
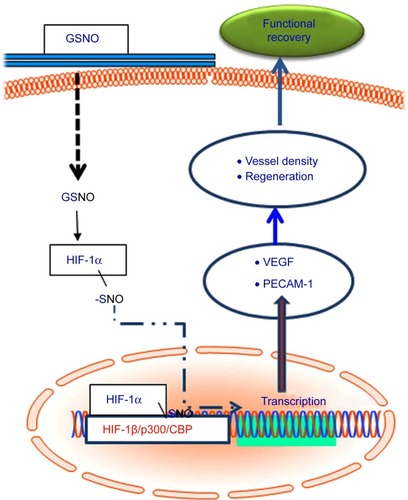
Figure 7 Schematic showing GSNO-mediated events leading to neuroprotection and neurorepair as well as functional recovery.