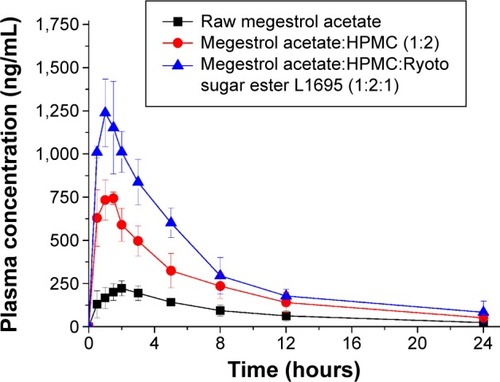
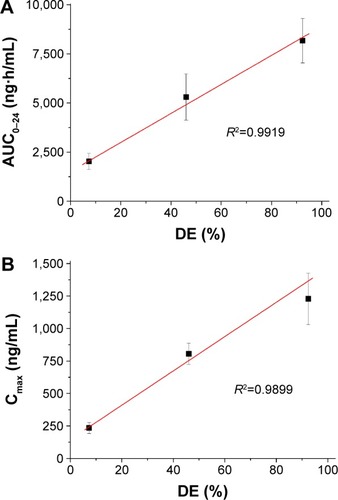
Figures & data
Table 1 The formulation, particle size, and specific surface area of the megestrol acetate solid dispersion nanoparticles prepared using the SAS process
Figure 1 Scanning electron micrographs (A), differential scanning calorimetry thermograms (B), powder X-ray diffraction patterns (C), and dissolution profiles (D) of megestrol acetate solid dispersion nanoparticles prepared using the SAS process.
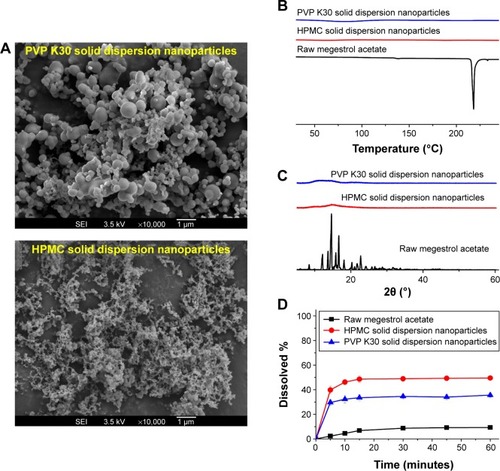
Figure 2 Scanning electron micrographs of megestrol acetate solid dispersion nanoparticles prepared using the SAS process.
Abbreviations: SAS, supercritical antisolvent; SEI, secondary electron imaging; HPMC, hydroxypropylmethyl cellulose; TPGS, D-α-tocopheryl polyethylene glycol 1000 succinate.
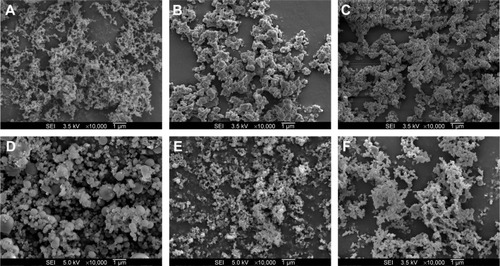
Figure 3 The differential scanning calorimetry thermograms of megestrol acetate solid dispersion nanoparticles prepared using the SAS process.
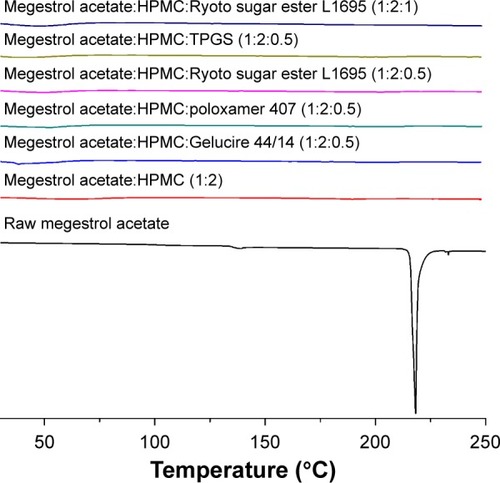
Figure 4 Powder X-ray diffraction patterns of megestrol acetate solid dispersion nanoparticles prepared using the SAS process.
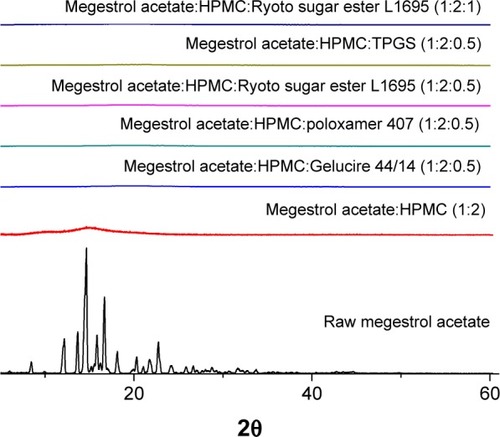
Table 2 The dissolution data of megestrol acetate solid dispersion nanoparticles prepared using the SAS process
Table 3 The solubility of megestrol acetate in various surfactant solutions at 1% concentration (w/v)
Figure 5 The dissolution profiles of megestrol acetate solid dispersion nanoparticles prepared using the SAS process.
Abbreviations: SAS, supercritical antisolvent; HPMC, hydroxypropylmethyl cellulose; TPGS, D-α-tocopheryl polyethylene glycol 1000 succinate.
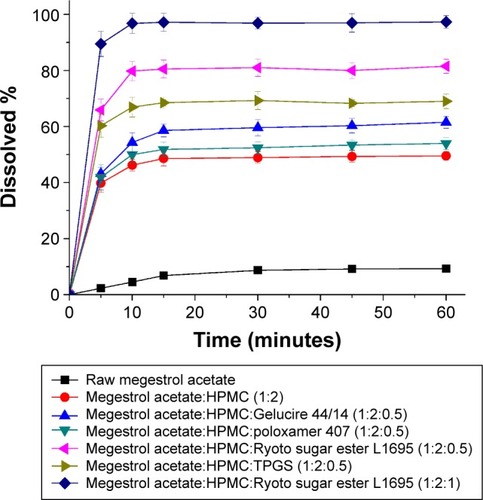
Table 4 Pharmacokinetic parameters of megestrol acetate solid dispersion nanoparticles prepared using the SAS process