Figures & data
Figure 1 Morphology of tested K9TCC and K9OSA cell lines.
Abbreviations: DOX, doxorubicin; K9OSA, canine osteosarcoma; K9TCC, canine transitional cell carcinoma.
Table 1 Doubling times for tested K9TCC and K9OSA cell lines
Table 2 IC50 values of DOX and AD198 of tested K9TCC and K9OSA cell lines
Figure 2 DOX- and AD198 (AD)-inhibited cell viability of tested K9TCC and K9OSA cell lines.
Abbreviations: AD, AD198; DOX, doxorubicin; K9OSA, canine osteosarcoma; K9TCC, canine transitional cell carcinoma; MTS, 3-(4,5-dimethyl-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
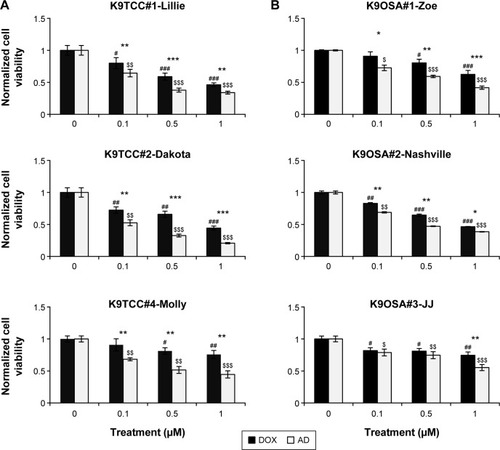
Figure 3 AD198 (AD)- and DOX-induced apoptosis and caspase activation in tested K9TCC and K9OSA cell lines in vitro.
Abbreviations: AD, AD198; PARP(CF), PARP-cleaved fragment; DOX, doxorubicin; K9OSA, canine osteosarcoma; K9TCC, canine transitional cell carcinoma; PARP, poly (ADP-ribose polymerase); PARP(T), total PARP; WB, Western blot.
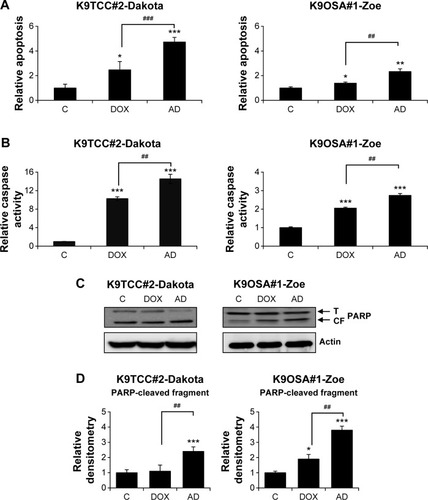
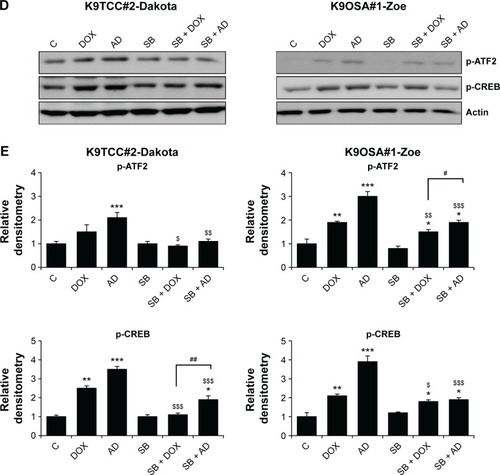
Figure 4 AD198 (AD) and DOX activated the PKC-δ pathway in tested K9TCC and K9OSA cell lines.
Abbreviations: AD, AD198; ATF2, activating transcription factor 2; PCK-δ (CF), cleaved fragment of PKC-δ; CREB, cyclic AMP response element binding protein; DOX, doxorubicin; K9OSA, canine osteosarcoma; K9TCC, canine transitional cell carcinoma; PKC-δ, protein kinase C-delta; WB, Western blot.
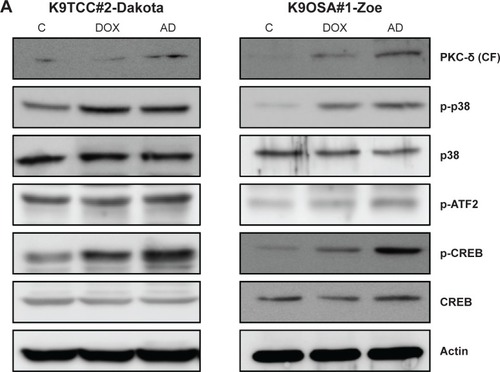
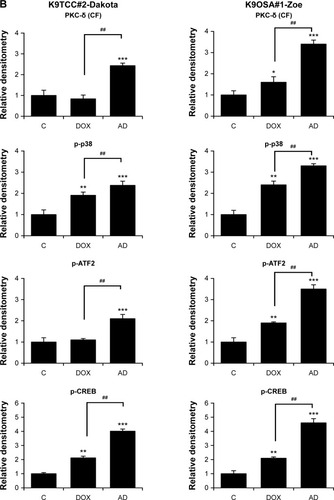
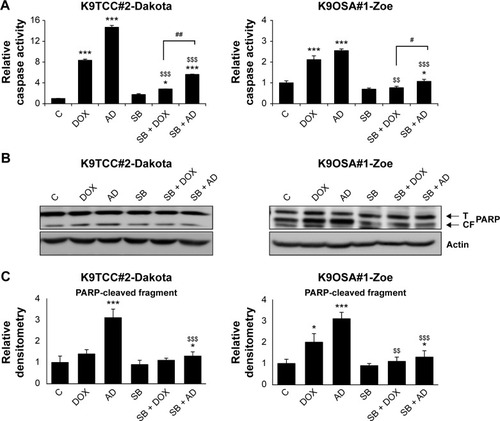
Figure 5 The inhibition of the p38 signaling pathway reduced AD198 (AD)- and DOX-induced apoptosis in tested K9TCC and K9OSA cell lines in vitro.
Abbreviations: AD, AD198; ATF2, activating transcription factor 2; PARP(CF), PARP cleaved fragment; CREB, cyclic AMP response element binding protein; DOX, doxorubicin; K9OSA, canine osteosarcoma; K9TCC, canine transitional cell carcinoma; PARP, poly (ADP-ribose) polymerase; SB, SB203580; PARP(T), total PARP; WB, western blot.