Figures & data
Figure 1 Study design.
Abbreviations: TAC, tacrolimus; MMF, mycophenolate mofetil.
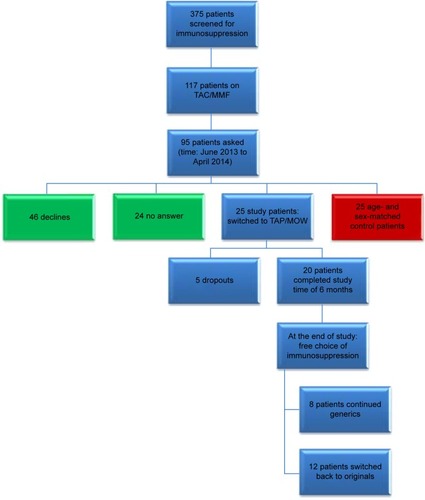
Table 1 Patient demographics
Figure 2 Matched-pair analysis of tacrolimus trough level/dose ratio.
Abbreviations: TAC, tacrolimus; MMF, mycophenolate mofetil.
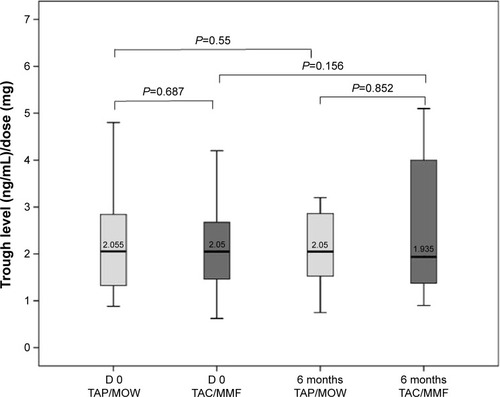
Figure 3 Change in tacrolimus trough level.
Abbreviations: TAC, tacrolimus; MMF, mycophenolate mofetil.
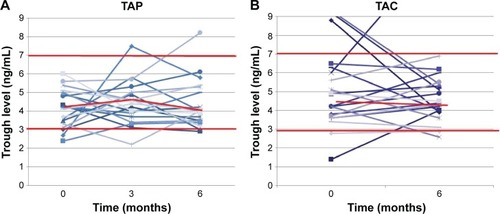
Table 2 Tacrolimus dose and dose adjustment (generic group)
Table 3 Tacrolimus dose and dose adjustment (original group)
Table 4 Matched-pair analysis of blood parameters
Table 5 Side effects
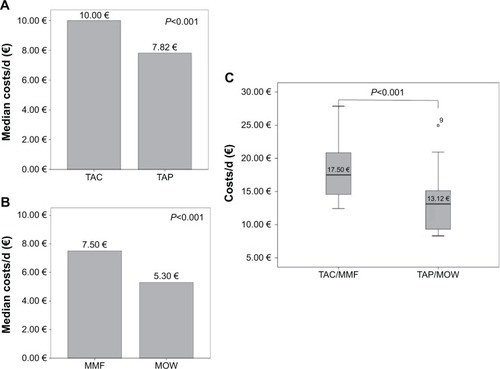
Figure 4 Intra-individual analysis of costs.
Abbreviations: TAC, tacrolimus; MMF, mycophenolate mofetil; d, day.