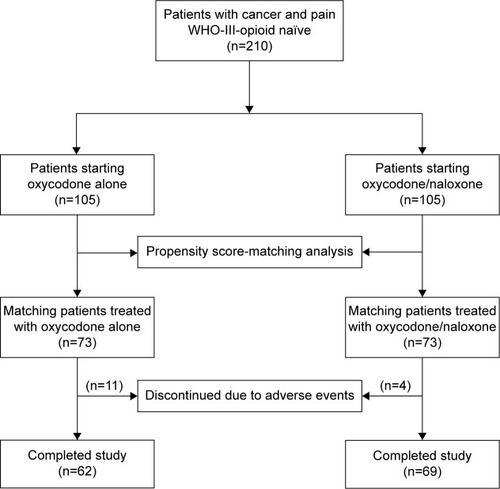
Figures & data
Table 1 Baseline characteristics of the study population
Figure 2 Average pain intensity (score on an 11-point NRS) during treatment with OXY and OXN.
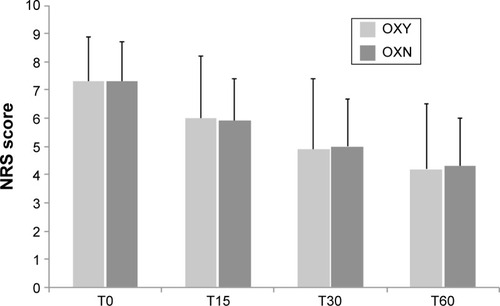
Figure 3 OXY and OXN mean daily dosages during the study.
Abbreviations: OXN, oxycodone/naloxone; OXY, oxycodone.
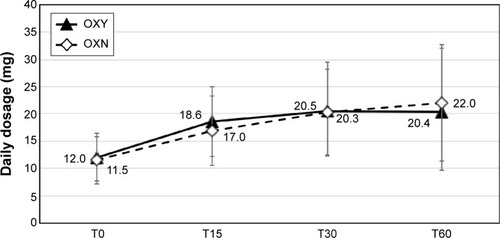
Table 2 Summary of quality of life endpoints and comparison between groups
Table 3 Summary of intestinal function endpoints and comparison between groups
Table 4 Summary of moderate-to-severe adverse drug reactions