Figures & data
Figure 1 The endothelin pathway. Endothelin-1 and the endothelin receptor pathway. Dual endothelin receptor antagonists, such as macitentan, block the binding of endothelin-1 to both the ETA and ETB receptor and block the downstream vasoconstricting effects. Reproduced from Lai YC, Potoka KC, Champion HC, et al. Pulmonary arterial hypertension: the clinical syndrome, Circulation Research, 115, 1, 115–130, https://www.ahajournals.org/.Citation15
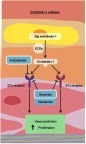
Table 1 Summary of clinical trials
Figure 2 Effect of macitentan on PAH-related hospitalizations. The effect of macitentan 10 mg daily, 3 mg daily, and placebo on the rate of hospitalization due to pulmonary arterial hypertension (PAH). Macitentan at 10 mg daily showed a 49.8% reduction in PAH-related hospitalizations compared to placebo. Reprinted from JACC Heart Fail, 3, 1, Channick RN, Delcroix M, Ghofrani HA, et al, Effect of macitentan on hospitalizations, 1–8, Copyright (2015), with permission from Elsevier.Citation25
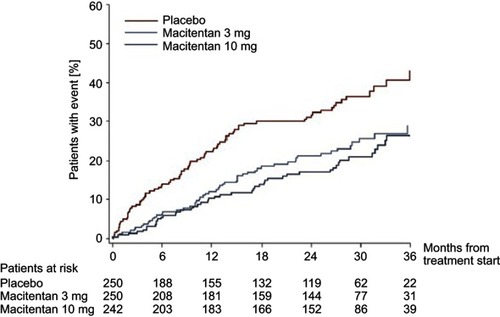
Figure 3 Time to significant decline in SF-36 score for macitentan (10 mg, 3 mg) vs placebo. Time to a significant decline in the SF-36 COMPONENT score. Overall, the 10 mg macitentan dose and 3 mg macitentan dose both significantly reduced the risk of a meaningful decline in the SF-36 scores until the end of treatment compared to placebo: Panel A displays the meaningful decline in the physical component score, Panel B depicts the meaningful decline in the mental component score. Reprinted from Chest, 151, 1, Mehta S, Sastry BKS, Souza R, et al, Macitentan improves health-related quality of life for patients with pulmonary arterial hypertension, 106–118, Copyright (2017), with permission from Elsevier.Citation27
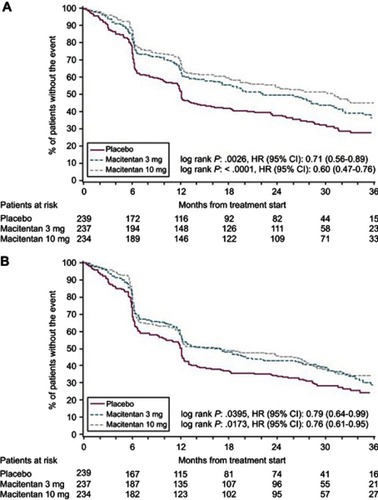
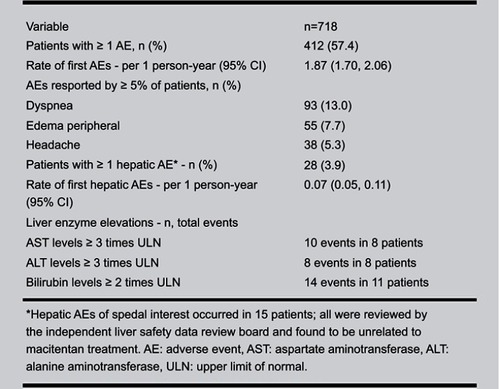
Figure 4 Adverse events, abnormalities in liver function tests and hemoglobin. Adverse events in the placebo, 3 mg, and 10 mg arms in the SERAPHIN trial, as well as rates of liver function abnormalities and anemia. From New England Journal of Medicine, Pulido T, Adzerikho I, Channick RN, et al, Macitentan and morbidity and mortality in pulmonary arterial hypertension, 369, 9, 809–818. Copyright © (2013) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.Citation5
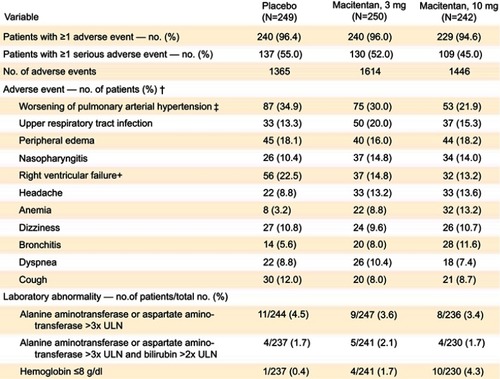
Figure 5 Rates of adverse events and liver function abnormalities with the use of macitentan in a real-world setting. Data from the OPUS registry evaluating adverse events and liver function test abnormalities with the use of 10 mg daily of macitentan in a real-world setting. Reprinted from The Journal of Heart and Lung Transplantation, 36, 4, Kim NH, Bergmark BA, Zelniker TA, et al, OPUS registry: safety and tolerability of macitentan in a real-world setting, S20-S21, Copyright (2017), with permission from Elsevier.Citation45