Figures & data
Table 1 Details of included studies
Figure 1 Incidences of constipation (all-causality), new-onset use of constipation remedies and discontinuations resulting from constipation during 12 weeks’ treatment with darifenacin 7.5 mg/day or 15 mg/day, tolterodine 4 mg/day or placebo in patients with overactive bladder.
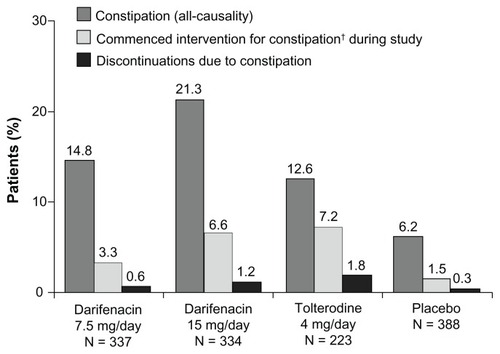
Table 2 Incidence of all-causality constipation in fixed-dose Phase III studies in OAB patients
Figure 2 Proportion of new cases of all-causality constipation reported at different time points during treatment with darifenacin 7.5 mg/day or 15 mg/day, tolterodine 4 mg/day or placebo.
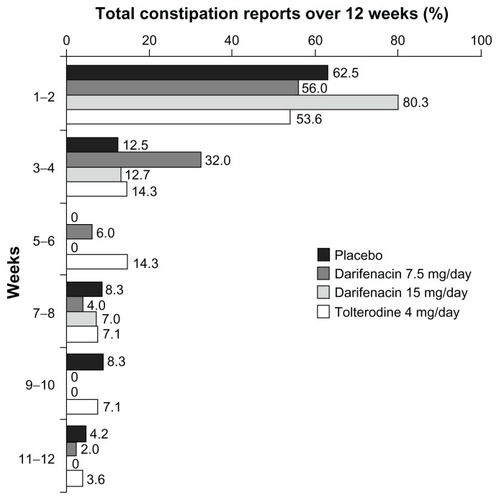
Table 3 Concomitant laxative use in fixed-dose Phase III studies in OAB patients
Figure 3 Patient perception of quality of treatment in fixed-dose Phase III studies in overactive bladder patients. (A) Patient satisfaction with drug; (B) Patient preference for study drug over previous therapy; (C) patient willingness to re-use study drug.
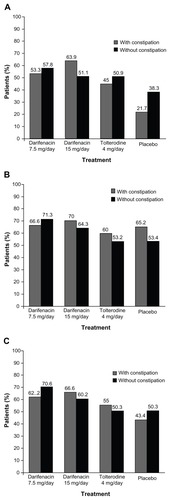
Table 4 Bowel habit questionnaires
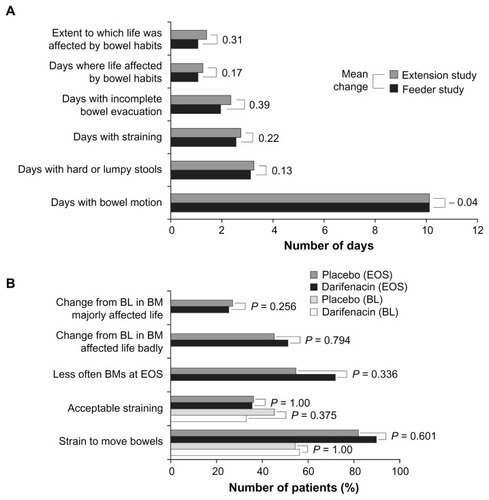
Figure 4 (A) Summary of bowel habit questionnaire responses in patients in a long-term 2-year study; (B) Summary of bowel habit questionnaire responses in patients aged ≥65 years; results relate to the last 2 weeks of treatment.
Abbreviations: BL, baseline; BM, bowel movements; EOS, end of study.