Figures & data
Table 1 Summary of Tafamidis Clinical Trials and Open-Label Extension Studies
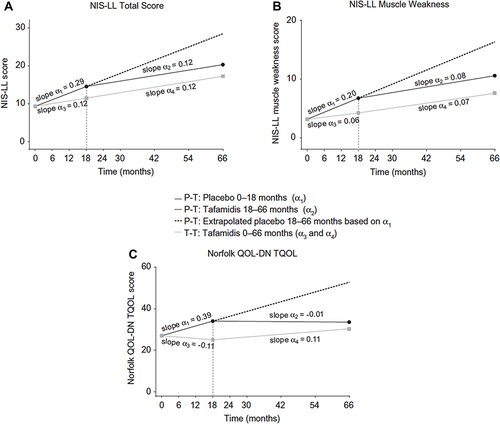
Figure 1 Intent-to-treat slope analysis of (A) NIS-LL total score, (B) NIS-LL muscle weakness, and (C) Norfolk QOL-DN in patients with Val30Met ATTRv-PN (data from Fx-005, Fx-006 and Fx1A-201).Citation17,Citation18,Citation20 Slopes are adjusted at mean baseline value of the two treatment groups.