Figures & data
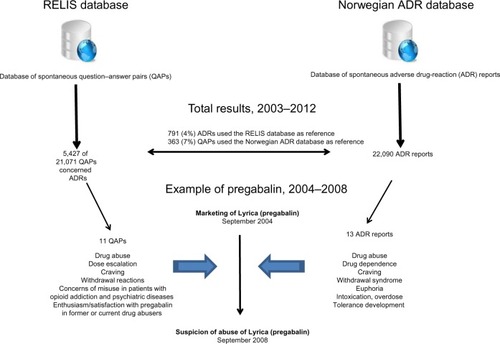
Figure 1 The Regional Medicines Information and Pharmacovigilance Centres (RELIS) in Norway provide feedback to health care professionals on spontaneous drug-related questions and adverse drug-reaction (ADR) reports published in a question–answer pair (QAP) database (the RELIS database) and the Norwegian ADR database, respectively.