Figures & data
Figure 1 Selection of study population.
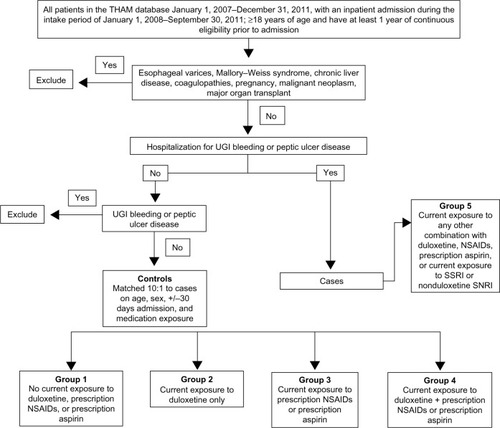
Table 1 Baseline demographics, comorbidity index, and concomitant drug prescriptions
Table 2 Study groups based on current exposure to prescription duloxetine, NSAIDs, or aspirin
Table 3 Doses of duloxetine prescribed across cases and controls
Table 4 UGI events across all cases and each dose of duloxetine in the duloxetine‐only exposure group
Figure 2 Adjusted OR and 95% CI for concomitant exposure to duloxetine, NSAIDs, and aspirin was not associated with an increased risk for UGI bleeding as compared with the risk from exposure to duloxetine only.
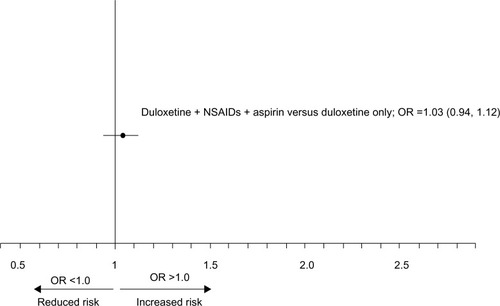
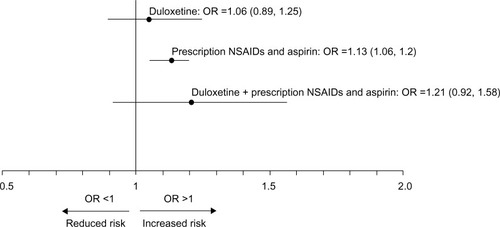
Figure 3 Adjusted OR (95% CI) for the risk of UGI in patients across medication exposure groups.
Abbreviations: OR, odds ratio; CI, confidence interval; UGI, upper gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs.