Figures & data
Figure 1 Consolidated Standards of Reporting Trials (CONSORT) diagram describing recruitment and retention of participants. If follow-up blood chemistry were missing, baseline values were carried forward.
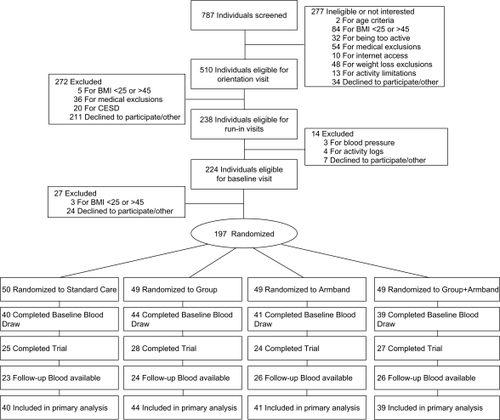
Table 1 Baseline characteristicsTable Footnote*
Table 2 Change in blood pressure and lipidsTable Footnote*
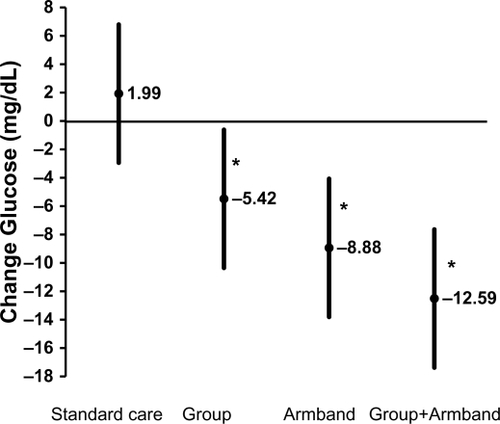
Figure 2 Mean change (least-squares mean ± 95% confidence interval) in fasting glucose for the standard care and intervention groups. Difference across groups were tested by analysis of covariance (ANCOVA) with adjustment for prespecified covariates (baseline age, gender, race, education, recruitment wave, and baseline glucose) among 152 participants not taking glucose medication. Significant ANCOVAs (P < 0.05) were followed by pair-wise comparisons to test whether intervention groups differed significantly from the standard care group.