Figures & data
Table 1 Classification of Partial Lipodystrophies, Prevalence and OMIM (Online Mendelian Inheritance in Man) Phenotype Number
Table 2 Clinical Features of Familial Partial Lipodystrophies
Table 3 Gene Mutations Involved in Familial Partial Lipodystrophy (FPLD) Pathogenesis, Mode of Inheritance and Corresponding Phenotypes
Figure 1 Pathways involved in the molecular pathogenesis of familial partial lipodystrophies. Responsible genes may affect adipocyte differentiation, cell membrane integrity, DNA repair, lipid droplet formation and lipolysis. PPARγ, the “master regulator” of adipogenesis coordinates the transcription of proteins central to the adipocyte function. AKT2 and PIK3R1 are involved in insulin signaling pathways, mediating adipocyte differentiation. LMNA gene encodes lamins A/C which are essential components of the nuclear envelope. ZMPSTE24 is responsible for the proteolysis of prelamin A to mature/active lamin A. PSM8 and PCYT1A are responsible for the composition and integrity of cell membranes. Mutations lead to intracellular oxidative stress, inflammation and apoptosis. WRN, POLD1 and BLM participate in DNA repair and replication, ensuring genomic stability. Caveolin 1, the product of CAV1, participates in the formation of caveolae. Caveolin vesicles are created from endocytosis of caveolae which carry cholesterol and sphingolipids from the cell surface. Caveolin vesicles are merged with the lipid droplet, mediating the transcytosis of fatty acids. CIDEC is responsible for the formation of unilocular lipid droplets and PLIN1 for the structure of the lipid droplets. Finally, LIPE and ADRA2A regulate triglyceride lipolysis to free fatty acids and glycerol.
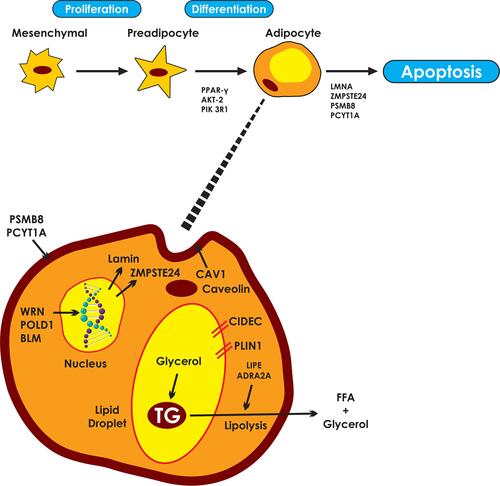
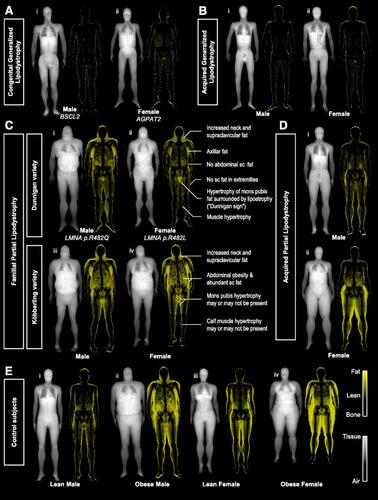
Figure 2 “Fat shadows”: reconstructed DXA scan images using color-coded representation to highlight adipose tissue. Patients with (A) congenital type 2 (i) and type 1 (ii) and (B) acquired generalized lipodystrophy have minimal residual fat depots. Patients with (C) FPLD of the Dunnigan (i and ii), and Köbberling variety (iii and iv), present with lipoatrophy of subcutaneous fat and hypertrophy of adipose tissue in the neck and upper trunk. Identification of the “Dunnigan sign” is diagnostic of FLPD2. Patients with acquired partial lipodistrophy (D) have loss of fat from the upper extremities and truncal region with lower half depots being either normal (i) or hypertrophied (ii). (E) Lean (i and iii) and obese (ii and iv) control subjects present gender-specific fat distribution. American Diabetes Association. “Fat Shadows” From DXA for the Qualitative Assessment of Lipodystrophy: When a Picture Is Worth a Thousand Numbers, Diabetes Care, 2018. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.Citation72