Figures & data
Figure 1 Evidence suggests that the mechanisms inhibiting both appetite and caloric ingestion may be impaired in obese individuals.Citation48
Abbreviations: CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; OXM, oxyntomodulin; PP, pancreatic polypeptide; PYY, peptide YY.
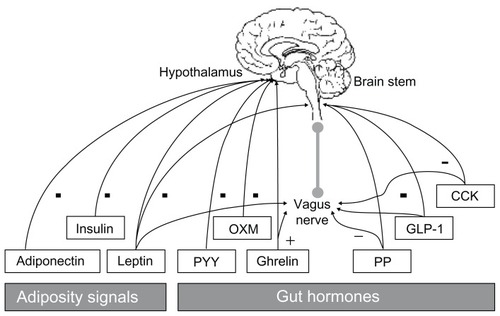
Figure 2 Postulated mechanisms for fatty acid control of gene transcription.Citation104 The FA per se, FA-CoA, or FA metabolite modulate (±) transcription of a responsive gene, encoding a protein involved in FA transport or metabolism, through various non-mutually selective potential mechanisms. Step 1: a signal transduction cascade is initiated to induce a covalent modification of a TF, thereby modifying its transcriptional potency. Step 2: the FA itself or its derivative acts as a ligand for a TF, which then can bind DNA at a FA response element and activate or repress transcription. Steps 3, 4 and 5: FA can act indirectly via alteration in either TF mRNA stability (Step 3) or gene transcription (Step 4), resulting in variations of de novo TF synthesis (Step 5) with an impact on the transcription rate of genes encoding proteins involved in FA transport or metabolism. On binding to the eognate response element, TF acts either as a monomer (Step 6), a homodimer, or a heterodimer with TF+, a different TF (Step 7).
Abbreviations: FA, fatty acids; FA-CoA, fatty acyl-CoA; TF, transcription factor.
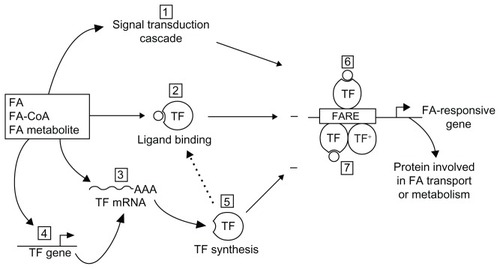
Table 1 Amino acid processes in muscle metabolism and their respective enzymes, yielding tricarboxylic acid cycle intermediates
Figure 3 Adaptation of muscle metabolism to a high availability of lipids.
Abbreviations: AA, amino acid; CHO, carbohydrate; CoA, coenzyme A; NH3, ammonia.
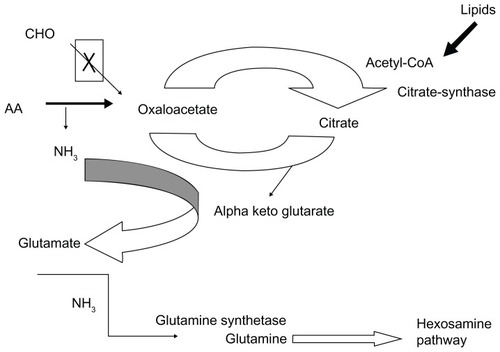
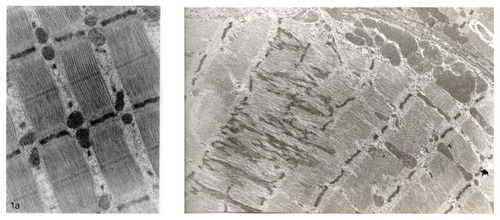
Figure 4 Micrograph of mitochondrial impairment caused by aspartate and asparagine supplementation in a rat model.