Figures & data
Figure 1 RSV suppressed LPS-induced RMCs’ proliferation as shown by MTT assay. Experiments were performed in triplicate with similar results. Data are means ± SEM. ##P<0.01 vs Control; *P<0.05 vs LPS-treated group, **P <0.01 vs LPS-treated group.
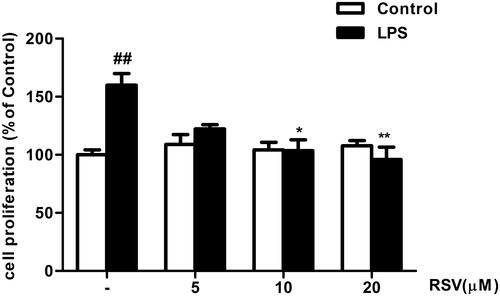
Figure 2 RSV decreased FN, ICAM-1 and iNOS expression in LPS-induced RMCs’ proliferation. MCs were cultured in DMEM with normal glucose (5.5 mM) containing 10% fetal bovine serum and then serum-free incubation for 24 h before pretreatment. MCs were stimulated by LPS (100 ng/mL) for different periods of time, from 0~48 h (A), and then FN, ICAM-1 and iNOS expression were detected by Western blot. After pretreatment with different concentrations of RSV (5, 10 and 20 µM) for 2 h, MCs were then treated with LPS (100 ng/mL) for another 24 h (B) or 12 h (C). Experiments were performed in triplicate with similar results. Data are means ± SEM. #P<0.05 vs Control, ##P<0.01 vs Control; *P<0.05 vs LPS-treated group, **P <0.01 vs LPS-treated group.
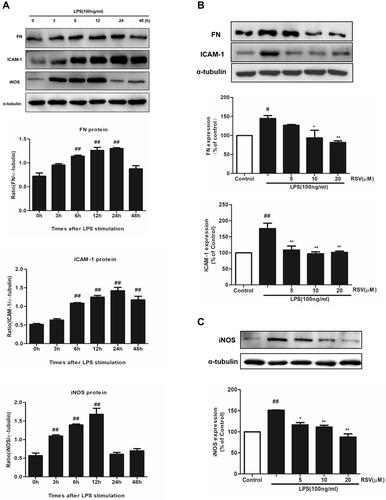
Figure 3 Effects of RSV on SphK1/S1P2 pathway in LPS-induced RMCs’ proliferation. RMCs were treated with the same method as above. (A) RMCs were stimulated by LPS (100 ng/mL) for different periods of time, from 0~48 h, and then the expression of SphK1 and S1P2 was measured by Western blot. (B) RMCs were stimulated with LPS for 24 h with or without RSV at 20 μM, SK-II or JTE-013. Then the expression of SphK1 and S1P2 were examined. (C and D) SphK1 activity and S1P content were measured in LPS-induced RMCs for 24 h with or without different concentrations of RSV, SK-II or JTE-013 by SphK activity assay kit and ELISA kit, respectively. (E) RMCs were stimulated with LPS for 24 h with or without RSV at 20 μM, SK-II or JTE-013. Then FN and ICAM-1 expression were examined. (F) RMCs were stimulated with LPS for 12 h with or without RSV at 20 μM, SK-II or JTE-013. Then iNOS expression was examined. Experiments were performed in triplicate with similar results. Data are means ± SEM. #P<0.05 vs Control, ##P<0.01 vs Control; *P<0.05 vs LPS-treated group, **P <0.01 vs LPS-treated group.
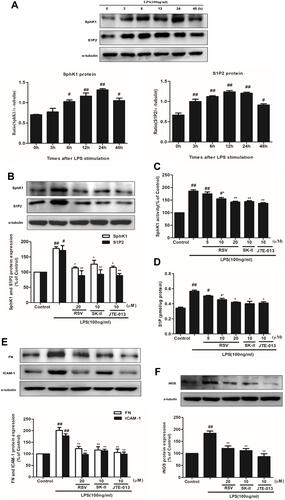
Figure 4 Effect of RSV on NF-κB activation in LPS-induced RMCs’ proliferation. RMCs were pretreated with RSV at the indicated concentrations or SK-II or JTE-013 for 2 h, followed by LPS stimulation for 30 min. (A) Nuclear extracts were subjected to EMSA to examine NF-κB DNA-binding activity. (B) NF-κB p65 expression in nucleus was examined by Western blot. Experiments were performed in triplicate with similar results. Data are means ± SEM. #P<0.05 vs Control, ##P<0.01 vs Control; *P<0.05 vs LPS-treated group, **P <0.01 vs LPS-treated group.
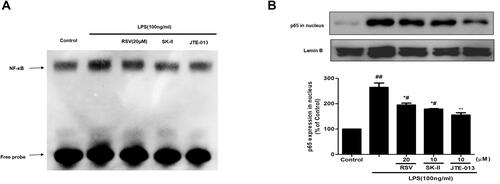
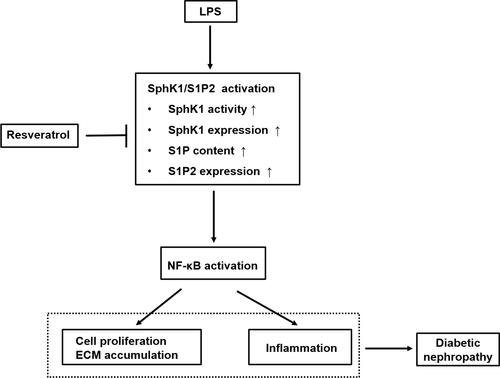
Figure 5 Schematic representation of the proposed underlying mechanism leading to renoprotective effect of RSV against LPS-mediated inflammation and mesangial cell proliferation in DN. This diagram summarizes our current study. RSV suppresses SphK1/S1P2 pathway activation in LPS-induced RMCs, which further reduces NF-κB activity. The inhibitory effects of RSV on SphK1/S1P2/NF-κB pathway may result from specific inhibition toward SphK1 and S1P2, then further inhibiting NF-κB pathway, thereby eventually exerting the renoprotective effects on DN, which is independent of its hypoglycemic effect.