Figures & data
Table 1 Demographic and baseline characteristics (safety population)
Table 2 Summary of adverse events (safety population)
Figure 2 Mean (± standard error) change in hemoglobin A1c(HbA1c) with colesevelam (3.75 g/day) versus a placebo in the double-blind phase (starting at baseline A) when added to metformin-based treatment in patients with inadequately controlled type 2 diabetes mellitus (safety population). In the open-label extension phase (starting at baseline B), all study participants received colesevelam (3.75 g/day).
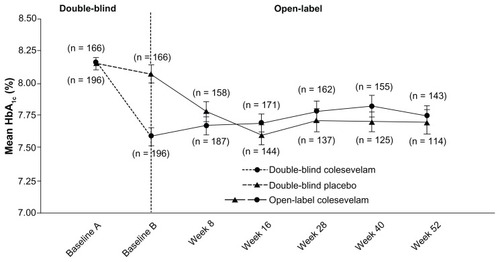
Figure 3 Mean (± standard error) change in fasting plasma glucose with colesevelam (3.75 g/day) versus the placebo in the double-blind phase (starting at baseline A) when added to metformin-based treatment in patients with inadequately controlled type 2 diabetes mellitus (safety population). In the open-label extension phase (starting at baseline B), all study participants received colesevelam (3.75 g/day).
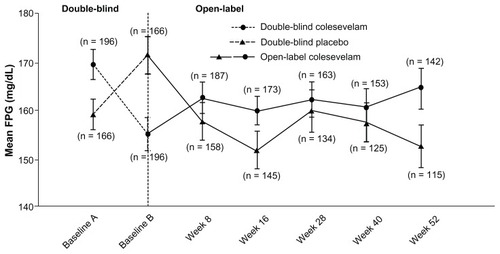
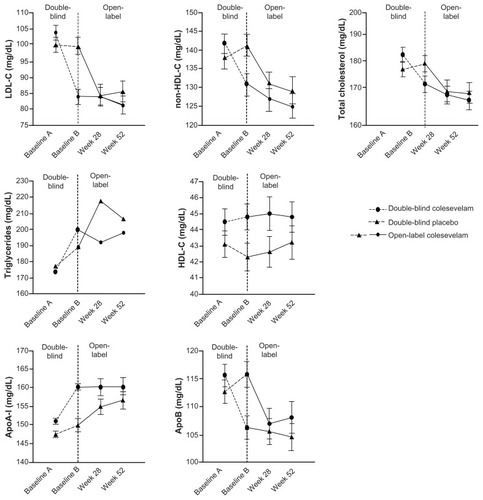
Figure 4 Mean (± standard error)* change in lipids and apolipoproteins with colesevelam (3.75 g/day) in the double-blind phase (starting at baseline A) when added to metformin-based treatment in patients with inadequately controlled type 2 diabetes mellitus (safety population). In the open-label extension phase (starting at baseline B), all study participants received colesevelam (3.75 g/day).
Abbreviations: Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.