Figures & data
Figure 1 Adverse effects of overweight and obesity on health such as major cardiovascular (CV) risk factors, including blood pressure, plasma lipids, glucose, inflammation, insulin resistance. Overweight is defined as BMI of 25.0 to 29.9 kg/m2. Obese is defined as BMI of 30 kg/m2 or higher.
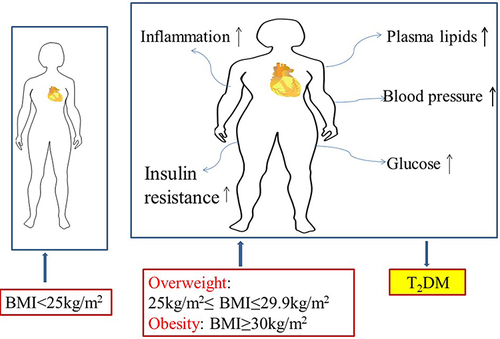
Figure 2 Insulin receptors in the kidney, liver, skeletal muscle and white adipose tissue. In skeletal muscle, insulin promotes glucose utilization and storage with the help of increasing glucose transport and net glycogen synthesis. In liver, insulin activates glycogen synthesis by increasing lipogenic gene expression and decreasing gluconeogenic gene expression. Insulin suppresses lipolysis and increases glucose transport and lipogenesis in white adipocyte tissue. In kidney, insulin participate in the RAAS, SNS activation, and the balance of sodium retention and renin activation.
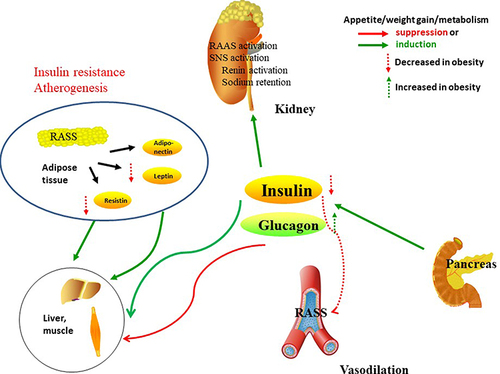
Figure 3 The human body adipose tissue can be divided into two main depots, subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). VAT in turn can be further classified into intrathoracic, abdominal and so on. Intrathoracic adipose tissue can be classified into epicardial adipose tissue (EAT) and pericardial adipose tissue based on its location within or outside the human pericardium respectively. Adipocytokine played significant roles in regulation of glucose, lipid and metabolism energy metabolism, which mediated insulin resistance in cardiovascular disease of type 2 diabetes including leptin. RBP4, chemerin, A-FABP, FGF21, fetuin-A, myostatin, IL-6, are the other adipokines, all of which may play significant roles in insulin sensitivity.
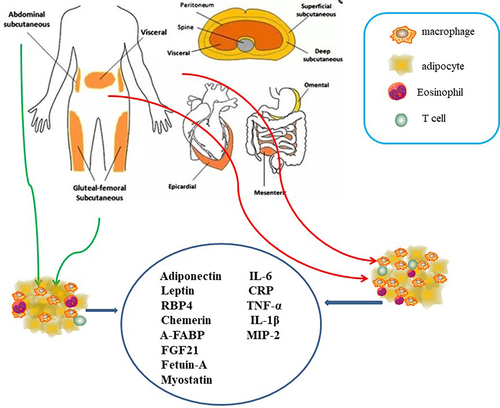
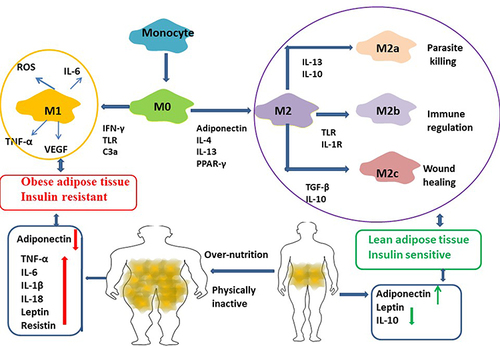
Figure 4 Macrophages (M0) were differentiated by human peripheral blood monocytes and then polarized to M1 and M2 phenotypes by using LPS/IFN-γ and IL-4/IL-13 respectively. Macrophages from lean adipose tissue are M2 phenotype, whereas in obese adipose tissue macrophages is M1 phenotype, expressing F4/80+CD11c+ and form crown‐like structures (CLS) surrounding the adipocytes. M1 macrophages mediate the metabolic complications, both in adipose tissue and by infiltration into other metabolic organs such as skeletal muscle. M2 macrophages can be divided into three major variants by different stimuli. M2a was elicited by IL-13 or IL-4. M2b was obtained by triggering Fc gamma receptors in the presence of a Toll receptor. M2c was elicited by IL-10, TGF-b or glucocorticoids. M2 macrophages express lower levels of inflammatory cytokines and higher levels anti-inflammatory cytokines. It participate in the lean adipose tissue insulin sensitive by the regulation of adiponectin, leptin and Il-10.