Figures & data
Table 1 Characteristics of Patients Who Suffered Gastrointestinal Adverse Drug Reactions When Using Semaglutide or Liraglutide
Table 2 RORs for Gastrointestinal Adverse Drug Reactions Upon Use of Semaglutide or Liraglutide
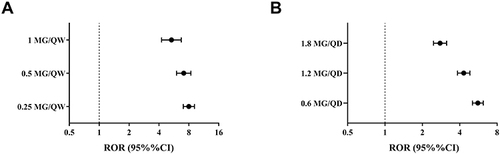
Figure 1 Differences in reporting of gastrointestinal adverse drug reactions between different GLP-1RAs as a radar chart. (A) Reporting-risk profile. (B) Time-to-onset profile. RORs (95% CIs) for reporting risk were calculated through a logarithmic transformation.
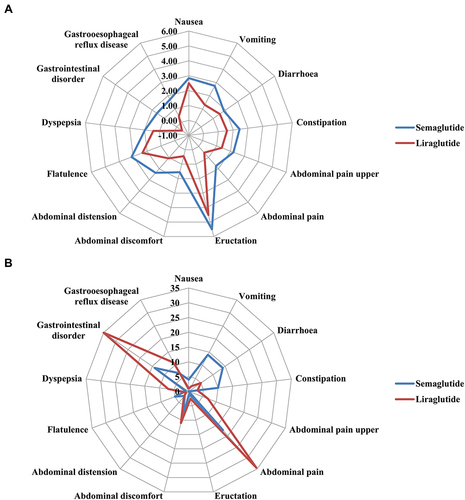
Table 3 Time-to-Onset of Cases with Semaglutide- or Liraglutide-Associated Gastrointestinal Adverse Drug Reactions