Figures & data
Figure 1 Structure of the BBB: capillary endothelial cell, tight junction, basal lamina, astrocyte, pericyte, interneuron.
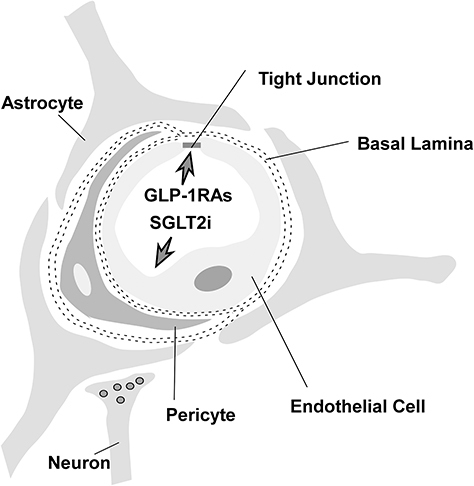
Figure 2 Mechanism of BBB transportation: a) Carrier-mediated influx, in which polar molecules are transported; b) Adsorptive-mediated transcytosis, in which positively charged macromolecules bind to receptors and are transported across the endothelial cell; c) Passive diffusion, in which most lipid-soluble molecules are transported; d) Receptor-mediated transcytosis, in which macromolecules bind to receptors and are transported to the CNS; e) Tight junction model (partially or completely open). Arrows indicate the direction of transportation.
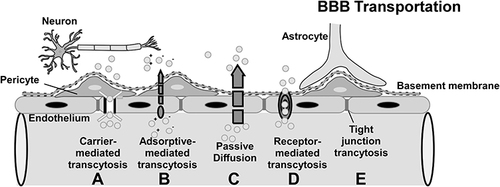
Table 1 The Physicochemical Properties of GLP-1 Receptor Agonists
Figure 3 Chemical structure and molecule weight of SGLT-2 inhibitors. Dapagliflozin(2013) MF:C21H25ClO6 MW:408.88; Empagliflozin(2014) MF:C23H27ClO7, MW:450.91; Sotagliflozin(2019) MF:C21H25ClO5S MW:424.94; Ertugliflozin(2017) MF:C22H25ClO7 MW:436.88; Luseogliflozin(2014) MF:C23H30O6S MW:434.55; Tofogliflozin(2014) MF:C22H26O6 MW:386.44; Ipragliflozin(2014) MF:C21H21FO5S MW:404.45; Canagliflozin(2013) MF:C24H25FO5S MW:444.52.
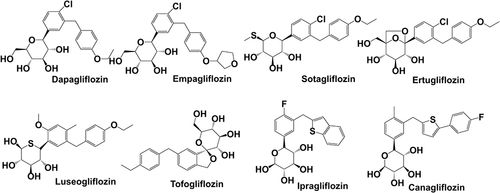
Table 2 Physicochemical properties related to penetration capacity of the BBB of SGLT-2is.
Figure 4 Factors influencing the blood-brain barrier permeability and possible treatment strategy. Increased oxidative stress, decreased VEGF levels and increased matrix metalloproteinase (MMP) activity result in basement membrane expansion, pericyte disintegration, increased paracellular diffusion, and decreased tight junction protein expression. In addition, fatty acids induce inflammatory responses, which reduce protein expression in BBB microvessels and increase BBB apoptosis, resulting in increased BBB permeability. Evidence shows that modification of the gut microbiota with prebiotics or probiotics and high-fiber diets improve systemic inflammation, restoring BBB integrity. In addition, treatment with GLP-1RAs and SGLT-2is inhibit the activation of inflammatory factors and attenuate oxidative stress via blood glucose and fatty acids reduction.
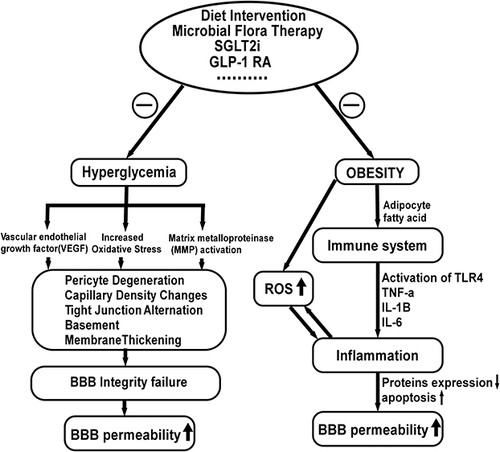
Figure 5 (A) Normal BBB: GLP-1RAs can cross the normal BBB by receptor-mediated transport and passive diffusion, but whether SGLT-2is may cross the compromised BBB via passive diffusion is still being investigated. (B) Impaired BBB.
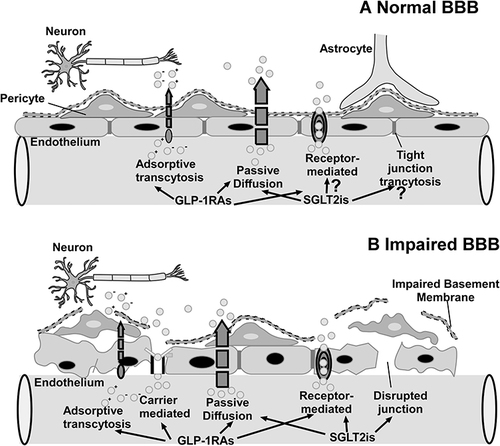
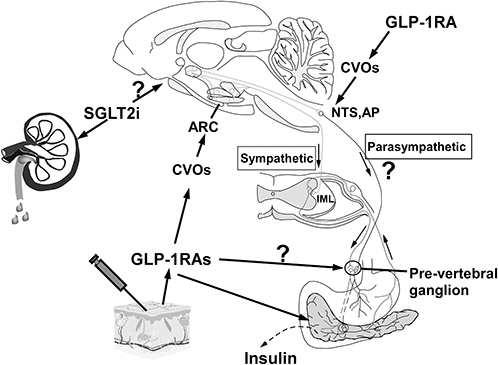
Figure 6 The arcuate nucleus (ARC) in the hypothalamus, the area postrema (AP) and the Nucleus of Solitary (NTS) in the brainstem are 3 examples of CVOs that are not protected by an effective blood-brain barrier. Once GLP-1RAs enter the bloodstream by subcutaneous injection, they can activate ARC hypothalamic POMC/CART neurons, which subsequently stimulate sympathetic efferent output, controlling insulin secretion. It is unclear if GLP-1-RAs stimulate the pre-vertebral ganglia directly. GLP-1RAs also activate GLP-1 receptors on the surface of pancreatic cells, resulting in the conversion of adenosine to cyclic adenosine phosphate (cAMP) in islet cells through conjugation of active G protein with adenosine cyclase. As cAMP levels rise, the pancreatic beta cell membrane depolarizes, which promotes insulin secretion. Further work is required to determine whether SGLT-2is may cross the BBB and exert similar effects on the brain.