Figures & data
Figure 1 Melanocortin system in glucose homeostasis. In the arcuate nucleus of the hypothalamus, there are many hormone receptors on pro-opiomelanocortin (POMC) neurons. Leptin, insulin, glucagon-like peptide (GLP)-1, and GLP-2 act on liver, skeletal muscle, and the pancreas to regulate blood glucose levels by binding with their corresponding receptors. At the same time, α-melanocyte stimulating hormone (α-MSH) on orexigenic or anorexigenic neurons binds with the melanocortin 4 receptor (MC4R) to regulate appetite and glucose homeostasis.
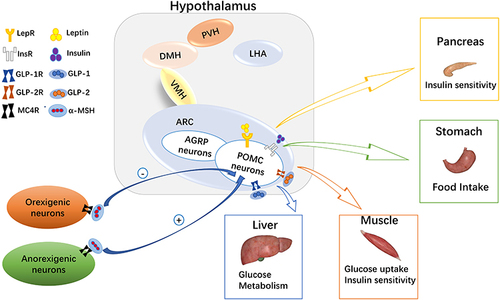
Figure 2 Leptin- and insulin-related signaling pathways in pro-opiomelanocortin (POMC) neurons. Leptin and insulin bind to their corresponding receptors and activate related signaling pathways, especially the phosphoinositide 3-kinase (PI3K) signaling pathway, which play important roles in glucose metabolism mediated by POMC neurons in the arcuate nucleus.
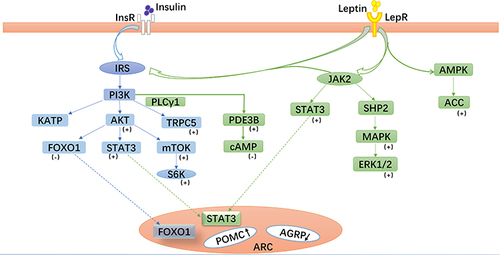
Figure 3 Toll-like receptor 4 (TLR4)-NF-κB pathway. Resistin can activate the TLR4-NF-κB pathway of pro-opiomelanocortin (POMC) neurons and inhibit expression of the insulin receptor, thereby regulating glucose homeostasis.
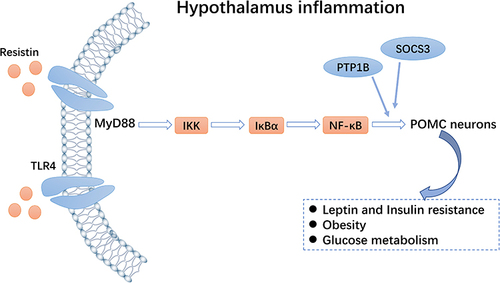
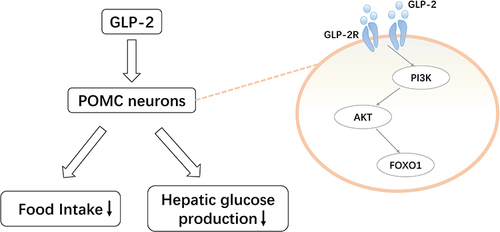
Figure 4 Pro-opiomelanocortin (POMC)-mediated regulation of glucagon-like peptide (GLP)-2 on glucose homeostasis. GLP-2 activates the phosphoinositide 3-kinase (PI3K)-AKT-forkhead box protein O1 (FOXO1) pathway by binding to the GLP-2 receptor expressed in POMC neurons. This reduces food intake and hepatic glucose production.