Figures & data
Table 1 Composition of the Jiang Tang San Huang Tablet
Table 2 Questionnaire Items for the Treatment of T2DM with JTSH Tablet
Figure 1 Flowchart of this study.
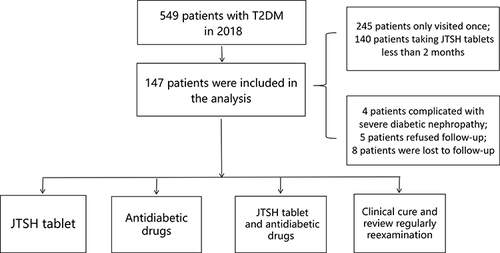
Table 3 The Distribution of Clinical Conditions of JTSH Tablet Users
Figure 2 Representations of the results of the questionnaire items. (A) Satisfaction of 147 patients’ with JTSH tablets treatment for T2DM. (B) The hypoglycemic regimens administered to the enrolled patients. (C) Evaluation of the advantages of JTSH tablets for treating of T2DM by lowering blood sugar or improving symptoms in 147 patients. (D) A total of 147 patients evaluated the disadvantages of JTSH tablets for the treatment of T2DM. (E) Evaluation of the hypoglycemic effect of antidiabetic drugs in 147 patients. (F) Evaluation of diet and exercise done well or not of 147 patients.
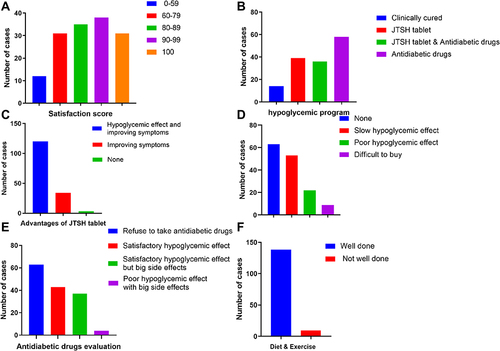
Figure 3 Hypoglycemic effect and islet protection effect of Jiang Tang San Huang tablets (JTSH tablets) in the treatment of type 2 diabetes mellitus. (A) Changes in glycosylated haemoglobin levels before and after treatment with JTSH tablet in 68 patients. (B) Changes in 2-hour postprandial blood glucose levels before and after treatment with JTSH tablets in 57 patients. (C) Changes in glycosylated haemoglobin levels before and after treatment with JTSH tablet in 43 patients. (D) Changes in HOMA-IR levels before and after treatment with JTSH tablet in 37 patients. (E) Changes in HOMA-β levels before and after treatment with JTSH tablet in 43 patients.
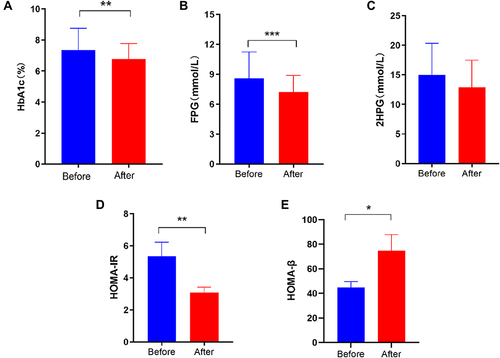
Table 4 Logistic Regression Between JTSH Tablet Administration and Glycaemic Control
