Figures & data
Table 1 Factors Induce Sympathetic Nervous System Overactivity and Insulin Resistance
Figure 1 The Summaries on referred non-neural pathways regarding the mechanism of SGLT2i in improving IR: 1. Glucotoxicity alleviation via increasing glycosuria, 2. Lipolysis increase and lipid content reduction, 3. Up-regulation of AMPK activity, 4. β-cell function improvement, 5. Oxidative stress mitigation, 6. Inflammation attenuation by reducing T cells and M1 macrophages accumulation. Up arrows: increase; down arrows: decrease.
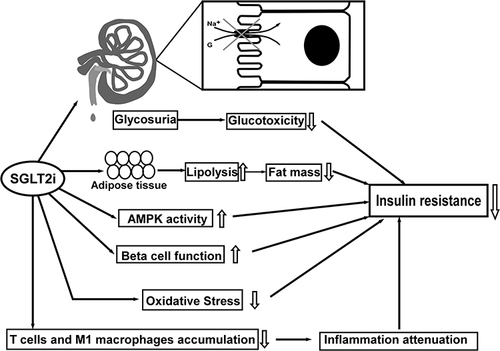
Figure 2 Scheme of the neural and metabolic mechanisms by which SGLT2i improves hepatic and adipose IR. SGLT2i triggers glycogen depletion signals in the liver, and liver may convey information to the CNS via the afferent vagus, activating efferent sympathetic nerves to adipose tissues, which promotes lipolysis leading to fat mass reduction. Meanwhile, activation of neurons in hypothalamus attenuates the glucose production and lipogenesis via efferent vagus to liver. SGLT2i could downregulate sympathetic activity innervating the liver by inhibiting expression of norepinephrine (NE) and NPY. SGLT2i activates the brain–adipose axis and induces fat mass loss. Both reduction of adipose tissue mass and hepatic glucose production could contribute to IR amelioration. Up arrows: increase; down arrows: decrease; (+): promote; (-): inhibit.
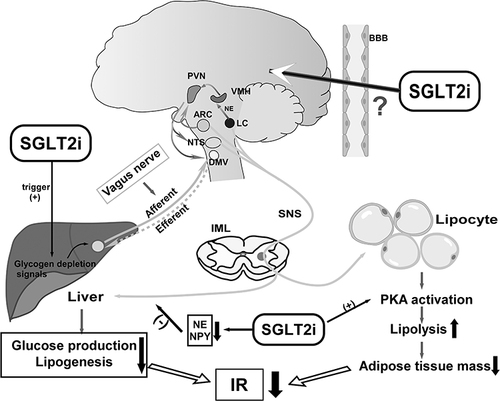
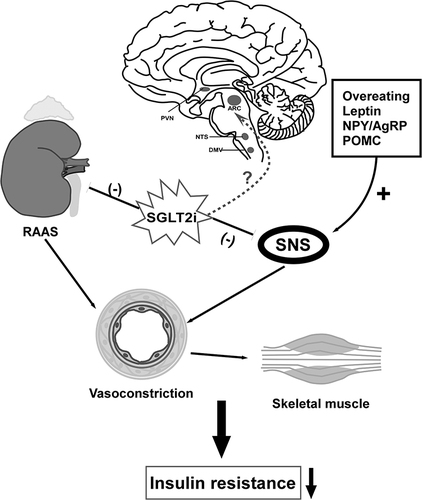
Figure 3 Regulation of neural pathway to attenuate muscular IR. Overeating and secretion of leptin increase sympathetic activity that induce IR of liver and skeletal muscle, so does overexpression of NPY. SGLT2i downregulates SNS by decreasing leptin, norepinephrine (NE) and NYP. SGLT-2i affect the sympathetic outflow of sympathetic preganglionic neurons to the intermediolateral nucleus of spinal cord (IML), thereby promoting parasympathetic activity of liver to improve hepatic glucose regulation and insulin sensitivity. Also, SGLT2i improves IR via suppression of renal RAAS component expression such as AT1R. Whether or to what extent SGLT2i act on neurons in the brain directly require further exploration. Down arrow: decrease; (-): inhibit; (+): promote.