Figures & data
Table 1 Baseline clinical and demographic characteristics of patients initiated on exenatide twice daily (BID) or insulin therapy for the total CHOICE cohortCitation27 and propensity-matched subgroup
Figure 1 Study disposition.
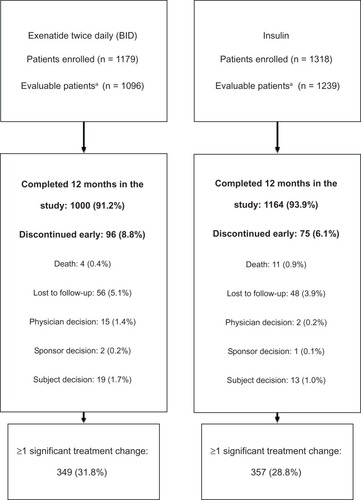
Figure 2 Kaplan–Meier estimates for time until significant treatment change after the initiation of first injectable therapy for the exenatide twice-daily (BID) cohort and total insulin cohort. The estimated number of patients remaining in the study with no significant treatment change is provided above each period.
Abbreviation: CI, confidence interval.
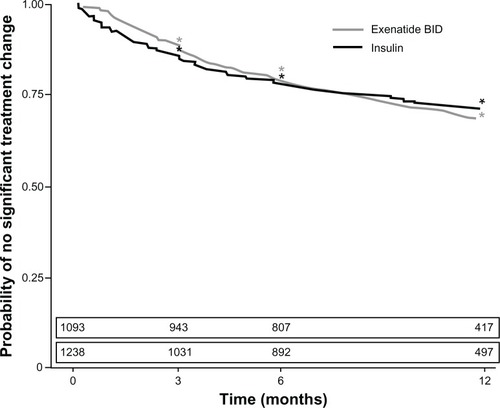
Table 2 Treatment change occurring during the 12 months following initiation of exenatide twice daily (BID) and insulin in patients with type 2 diabetes mellitusTable Footnote a
Table 3 Mean changes in clinical variables from baseline to 12 months (for patients with data at 12 months) after initiation of exenatide twice daily (BID) or insulin in patients with type 2 diabetes mellitus – initiators population
Table 4 Hypoglycemia occurring between baseline and 12 months (or time of study discontinuation, if earlier than 12 months) after initiation of exenatide twice daily (BID) or insulin in patients with type 2 diabetes mellitus – initiators population
Table 5 Gastrointestinal (GI) events occurring during the 12 months (or time of study discontinuation, if earlier than 12 months) following initiation of exenatide twice daily (BID) and insulin in patients with type 2 diabetes mellitus – initiators population
Figure 3 The incidence of gastrointestinal (GI) events after initiation of exenatide twice daily (BID) or insulin in patients with type 2 diabetes mellitus.
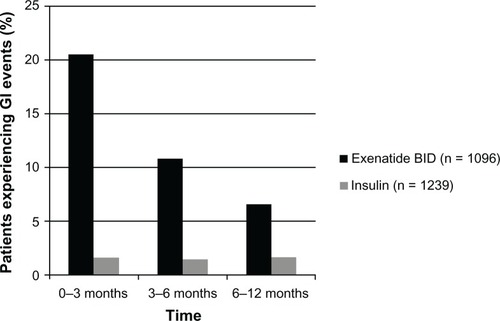
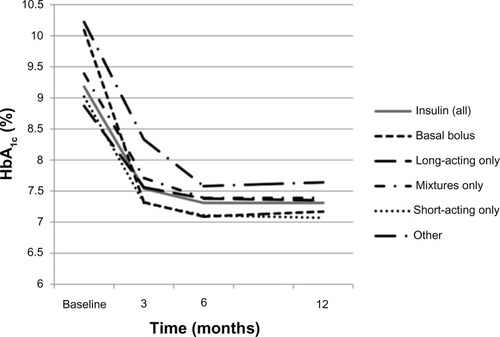
Figure 4 Change in glycated hemoglobin (HbA1c) according to the type of first insulin therapy initiated during the 12 months following initiation of that therapy.