Figures & data
Table 1 Related Fusion Protein Analogs for the Treatment of Diabetes
Figure 1 The signaling pathways and targets of drug action in normal humans after glucose uptake. In normal humans, blood glucose rises after food intakes and stimulates insulin secretion from pancreatic beta cells. Insulin first binds to the insulin receptor (IR) on the cell surface, which phosphorylates the insulin receptor itself, and the activated IR promotes phosphorylation of the tyrosine structural domain on the insulin receptor substrate (IRS), followed by signal transfer to the downstream PI3K/Akt2 pathway to amplify its active effect, and the activated Akt in turn continues to phosphorylate downstream effector molecules, including Rab-GTPase-activating protein, which ultimately translocates glucose transporter 4 (Glut-4)-vesicles from the cytosol to the cell membrane surface, regulating cellular glucose uptake. GLP-1 is known to inhibit glucagon secretion and promote insulin secretion. Dulaglutide, Albiglutide and Efpeglenatide, which are GLP-1 RAs, can lower blood glucose through both processes. Il-6 and TNF-α activation decreases the tyrosine kinase activity of insulin and impedes insulin-mediated glucose uptake in skeletal muscle and glucose uptake by adipocytes, both corresponding to the antagonists Olamkicept and Etanercept, which can block this pathway to achieve a lowering effect on blood glucose.
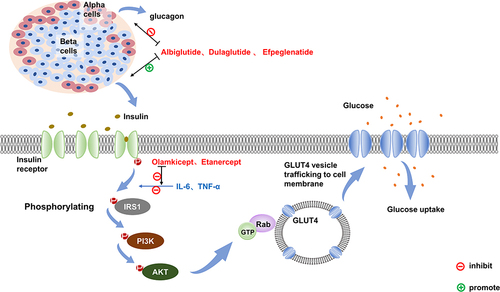
Table 2 Types of Long-Acting Drugs
