Figures & data
Figure 1 Amino acid sequence of GLP-1R/GIPR coagonist, DR10627 and the related peptides GLP-1, GIP, exenatide and liraglutide. Differences in amino acids from native GLP-1 and GIP are denoted in red. K* indicates a palmitoyl group conjugated to the ε-N of K10 via a -γ-glutamyl-linker (γE-C16) (A). Structure schematic of DR10627 (B).
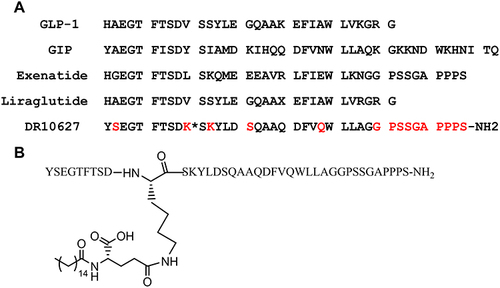
Figure 2 Representative concentration–response curves of DR10627, liraglutide, GLP-1 or GIP in cAMP accumulation assays of CHO cell lines expressing human GLP-1 receptors (A) or human GIP receptors (B). Values are presented as the mean (± SD) from duplicate analyses fitted with a 4-parameter logistic fit to determine the EC50 value.
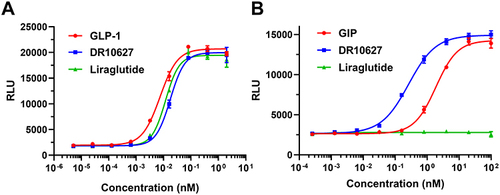
Table 1 Pharmacokinetic Parameters in Cynomolgus Monkeys (Mean ± Standard Deviation)
Figure 3 Pharmacokinetics of DR10627 in cynomolgus monkeys. Blood samples were collected at the indicated time points after a single s.c. injection of DR10627 (1 nmol/kg, 5 nmol/kg, 26 nmol/kg) in cynomolgus monkeys (n = 6). The serum concentrations of DR10627 were measured by LC-MS/MS. Pharmacokinetic parameters of DR10627 were calculated with Phoenix WinNonlin 8.1 using the method of non-atrioventricular model analysis. Values are presented as mean (± SD).
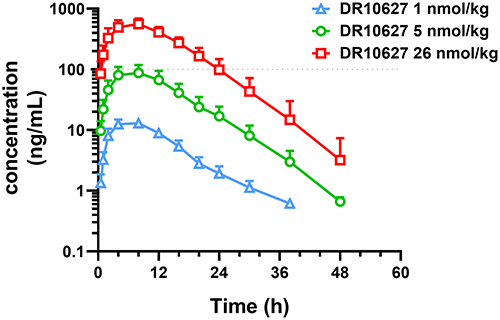
Figure 4 Effects of single doses of DR10627 and liraglutide on glucose tolerance in OGTT in SD rats. Glucose levels of rats at 0, 15, 30, 60, 90 and 120 min post-glucose challenge (A) and overall area under the curve (AUC) values (B). Male SD rat fasted overnight were dosed orally with D-glucose solution at a dose of 2 g glucose/kg of animal body weight following subcutaneous injection of the respective doses of vehicle, liraglutide (50 nmol/kg) or DR10627 (1 nmol/kg, 4 nmol/kg, 12 nmol/kg). Blood samples were collected at the indicated time points. Time 0 was immediately prior to glucose challenge. The values represent the mean ± SD. n = 12 mice/group. ***p < 0.001 compared to vehicle; &p < 0.05 compared to liraglutide (50 nmol/kg).
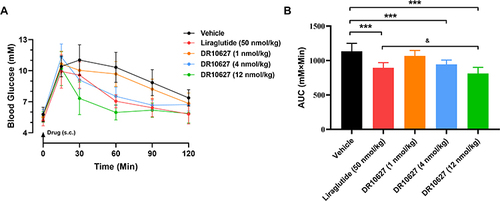
Figure 5 Effects of repeated-dose treatment with DR10627 and liraglutide on glucose tolerance in db/db mice. Male db/db mice were treated with either a daily s.c. administration the respective doses of vehicle, liraglutide (50 nmol/kg) or DR10627 (2 nmol/kg, 6 nmol/kg, 18 nmol/kg) for 4 weeks. Blood glucose was measured before administration to 24 h after administration on D1 (A) and D28 (B), random blood glucose was measured on D6, D13 and D20 (C). Fasting blood glucose was measured on D7, D14, D20 and D25 (D). The values represent the mean ± SD. n = 10 mice/group.
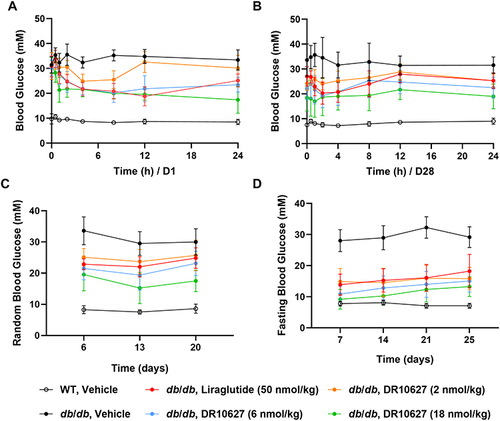
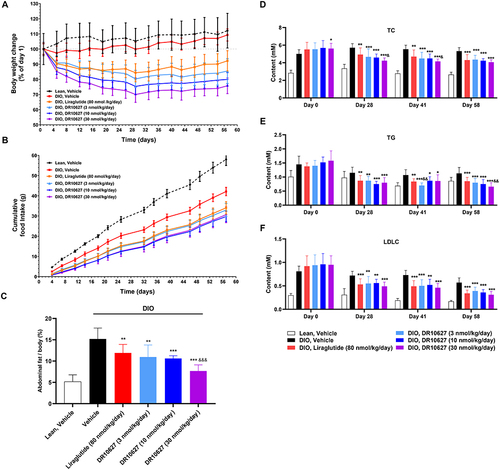
Figure 6 Effects of 11 weeks of repeated-dose treatment with DR10627 on body weight change (A), cumulative food intake (B), abdominal fat to body weight ratio (C), cholesterol (D), triglyceride (E) and LDLC (F) in male DIO mice. Male DIO mice were treated with either a daily s.c. administration the respective doses of vehicle, liraglutide (80 nmol/kg) or DR10627 (3 nmol/kg, 10 nmol/kg, 30 nmol/kg) for 11 weeks. Abdominal brown fat was harvested after the final blood sample collection. Blood samples were collected on D0 (pre-dose), D28, D41 and D48 from the tail veins of mice fasted overnight. The values represent the mean ± SD. n = 10 mice/group. *p < 0.05, **p < 0.01, ***p < 0.001 compared to vehicle; &p < 0.05; &&p < 0.01 compared to liraglutide (80 nmol/kg).