Figures & data
Figure 1 Pharmacokinetics of EQW. Plasma exenatide concentrations following a single dose of EBID (n=39) and multiple doses of EQW (n=31).
Modified from Fineman et al. with kind permission from Springer Science+Business Media: Clin Pharmacokinet, Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing, 2011;50(1):65–74, Fineman M, Flanagan S, Taylor K, et al., .Citation32
Abbreviations: BID, twice daily; EBID, exenatide twice daily; EQW, exenatide once weekly; QW, once weekly; SD, standard deviation.
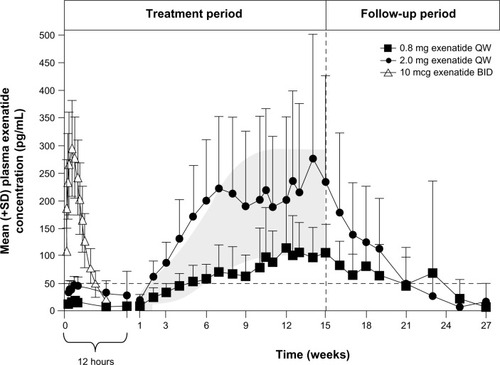
Table 1 Summary of EQW outcomes in the DURATION study program
Figure 2 Effects of EQW on glycemia and weight relative to sitagliptin in the DURATION study program.
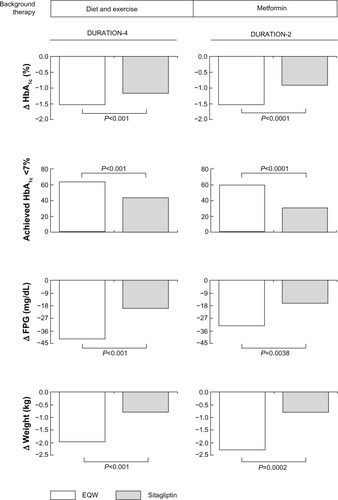
Figure 3 Effects of EQW on glycemia and weight relative to comparators in the DURATION trials.
Abbreviations: D/E, diet and exercise; EBID, exenatide twice daily; EQW, exenatide once weekly; HbA1c, glycated hemoglobin; MET, metformin; SFU, sulfonylurea; TZD, thiazolidinedione; DURATION, Diabetes Therapy Utilization: Researching Changes in A1c, Weight and Other Factors Through Intervention with Exenatide Once Weekly.
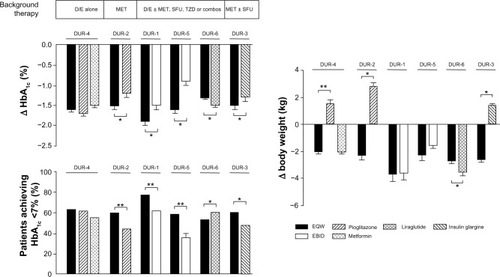
Table 2 Rates of gastrointestinal adverse events associated with incretin therapies in the DURATION study program