Figures & data
Figure 1 Chemical structure of TRC210258: N-(4-chlorophenyl)-2-(4-fluoro phenoxy)-N-methylimidazo [1, 2-a] pyrimidine-3-carboxamide.
![Figure 1 Chemical structure of TRC210258: N-(4-chlorophenyl)-2-(4-fluoro phenoxy)-N-methylimidazo [1, 2-a] pyrimidine-3-carboxamide.](/cms/asset/9871e13a-0f8f-4bf9-a857-7a302ab8380a/dmso_a_50209_f0001_b.jpg)
Figure 2 (A) Schematic representation of study design for in vivo efficacy experiment A to evaluate the effect of treatment with TRC210258 on energy expenditure in DIO mice. High-fat DIO C57bl/6J mice were randomized into two treatment groups. Mice in group 1 (n=5) received vehicle (0.5% w/v Tween-80 + 0.5% w/v Na-CMC solution in water) throughout the study. Group 2 (n=5) received TRC210258 2.3 mg/kg for the first 4 days. On day 5, the dose of TRC210258 was increased to 4.5 mg/kg, and on day 9 dose was increased further to 9.0 mg/kg. All treatments were administered once a day via the oral route. Oxygen consumption was recorded using the Oxymax® system (Columbus Instruments, Columbus, OH, USA) on day 4 of the respective incremental dose. (B) Schematic representation of study design for in vivo efficacy experiment B, which investigated the effect of TRC210258 on glycemic control in DIO mice. High-fat DIO C57bl/6J mice were randomized into three treatment groups. Animals in group 1 (n=10) received vehicle (0.5% w/v Tween-80 + 0.5% w/v Na-CMC solution in water), those in group 2 (n=9) received TRC210258 9 mg/kg, and those in group 3 (n=9) received sitagliptin 1 mg/kg. All treatments were administered once a day via the oral route. (C) Schematic representation of the design for the in vivo efficacy experiment studying the effect of TRC210258 on dyslipidemic parameters and triglyceride clearance in the high fat-fed DIO hamster. Male Golden Syrian hamsters with DIO were randomized into three treatment groups. Hamsters in group 1 (n=10) received vehicle (0.9% Na-CMC + 0.1% Tween-80 in phosphate-buffered saline, pH 7.4) of TRC210258 by the intraperitoneal route, and the vehicle (0.5% w/v Tween-80 + 0.5% w/v Na-CMC in water) for sitagliptin by the oral route; group 2 (n=10) received TRC210258 6 mg/kg by the intraperitoneal route and vehicle for sitagliptin by the oral route; group 3 (n=9) received sitagliptin 3 mg/kg by the oral route and vehicle for TRC210258 by the intraperitoneal route.
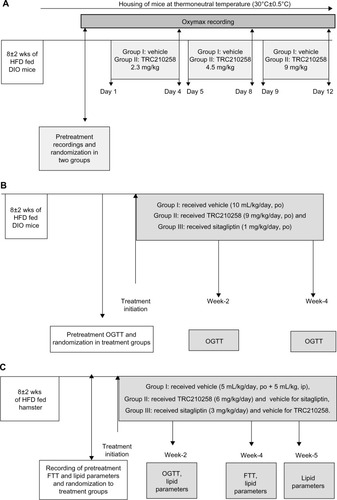
Figure 3 Activation of TGR5 by TRC210258. (A) Effect of TRC210258 on TGR5-mediated cAMP formation. EC50 value expressed in nM. (B) Effect of TRC210258 on CRE luciferase reporter. EC50 value expressed in nM. (C) Effect of TRC210258 on GLP1 secretion from NCI-H716, a human enteroendocrine cell line. GLP1 levels expressed in pM. Each value is the average of two experiments, each performed in duplicate. *P<0.05, TRC210258 versus vehicle control.
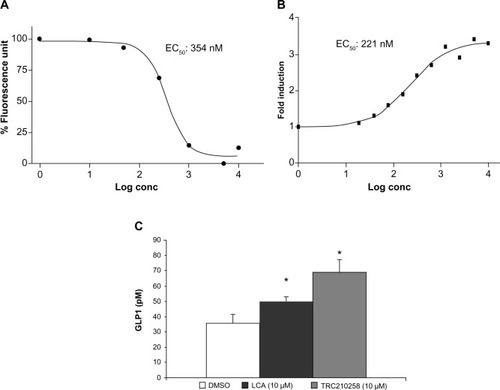
Figure 4 Effect of TRC210258 on energy expenditure in DIO mice. (A) The oxygen consumption profile was measured for ~22 hours (started at 9.0 am) on day 4 of treatment with different doses of TRC210258 or vehicle. #P<0.05, 4.5 mg/kg group versus respective vehicle group; *P<0.05, 9 mg/kg group versus respective vehicle group. (B) Average oxygen consumption during initial 5 hours post-drug administration (shaded area, right Y-axis scale) and the total 22 hours of recording (nonshaded area, left Y-axis scale) on treatment with different doses of TRC210258 or vehicle. *P<0.05 for TRC210258 9 mg/kg versus vehicle control group; **P<0.01 for TRC210258 9 mg/kg group versus vehicle control group & #P<0.05 for TRC210258 4.5 mg/kg group versus vehicle control group.
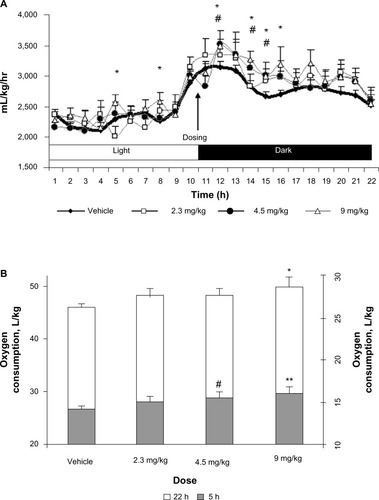
Figure 5 Effect of TRC210258 on glycemic parameters in DIO mice. (A) Bar graph represents the average area under the curve (AUC0–120 min) for plasma glucose during an oral glucose tolerance test at pretreatment basal level and after 2 and 4 weeks of treatment. (B) Plasma glucose profile during oral glucose tolerance test after 4 weeks of treatment. (C) Fasting plasma glucose (left y axis) and insulin (right y axis) after 4 weeks of treatment. (D) HOMA-IR after 4 weeks of treatment. *P<0.05 for treatment group versus vehicle control group; **P<0.01 for treatment group versus vehicle control group.
Figure 6 Effect of TRC210258 on plasma lipid parameters in DIO hamsters. (A) Total cholesterol, (B) LDL cholesterol, (C) non-HDL cholesterol, and (D) remnant cholesterol, in TRC210258, sitagliptin, and vehicle control groups at pretreatment basal level and after weeks 2, 4, and 5 of treatment. *P<0.05, **P<0.01, and ***P<0.001 for TRC210258 group versus vehicle group; #P<0.05 and ##P<0.01 for TRC210258 group versus sitagliptin group.
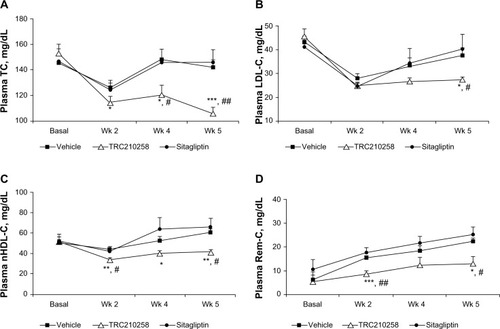
Figure 7 Effect of TRC210258 on plasma triglyceride clearance in DIO hamsters. (A) Incremental plasma triglyceride profile after an olive oil load in the TRC210258, sitagliptin, and vehicle control groups during fat tolerance testing after 4 weeks of treatment. *P<0.05, for TRC210258 group compared at multiple time points with the vehicle group. (B) Bar graph represents the mean AUC for plasma triglycerides during the fat tolerance test. *P<0.05 for TRC210258 group versus vehicle control group.
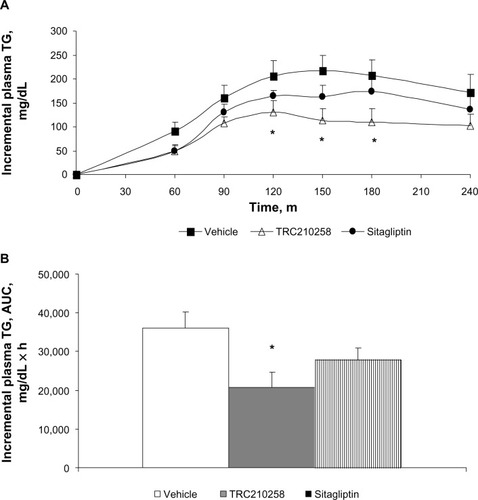
Figure 8 Effect of TRC210258 on glycemic parameters in DIO hamsters: (A) Plasma glucose profile during oral glucose tolerance test after 4 weeks of treatment (B) AUC(0–120 min) plasma glucose (left y axis) and insulin (right y axis) after 4 weeks of treatment in DIO hamsters. **P<0.05 versus vehicle control group.
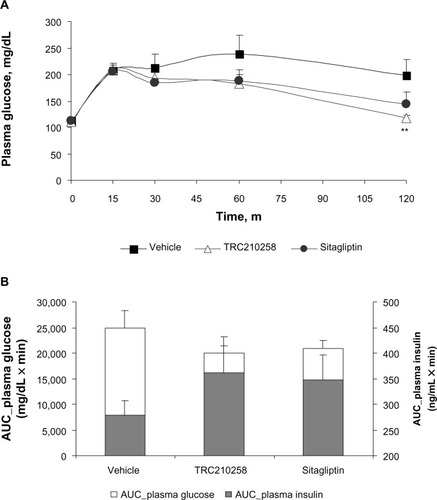
Figure 9 Schematic representation summarizing TRC210258-mediated TGR5 activation and improvement of glycemic and dyslipidemic cardiovascular risk.
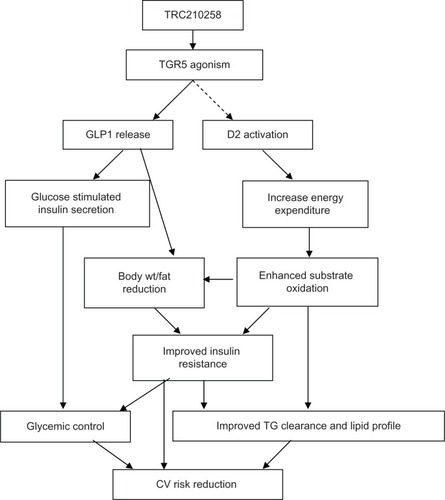
Table S1 Characteristics of the high-fat DIO hamster model
Table S2 Pharmacokinetic data for TRC210258 in DIO mice (9 mg/kg orally) and DIO hamsters (6 mg/kg, intraperitoneally)
